- Record: found
- Abstract: found
- Article: found
Possible Role of Adenosine in COVID-19 Pathogenesis and Therapeutic Opportunities
Read this article at
Abstract
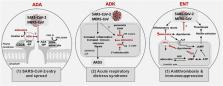
The outbreak of the novel coronavirus disease 2019 (COVID-19) caused by Severe Acute Respiratory Syndrome CoronaVirus-2 (SARS-CoV-2) requires urgent clinical interventions. Crucial clinical needs are: 1) prevention of infection and spread of the virus within lung epithelia and between people, 2) attenuation of excessive lung injury in Advanced Respiratory Distress Syndrome, which develops during the end stage of the disease, and 3) prevention of thrombosis associated with SARS-CoV-2 infection. Adenosine and the key adenosine regulators adenosine deaminase (ADA), adenosine kinase (ADK), and equilibrative nucleoside transporter 1 may play a role in COVID-19 pathogenesis. Here, we highlight 1) the non-enzymatic role of ADA by which it might out-compete the virus (SARS-CoV-2) for binding to the CD26 receptor, 2) the enzymatic roles of ADK and ADA to increase adenosine levels and ameliorate Advanced Respiratory Distress Syndrome, and 3) inhibition of adenosine transporters to reduce platelet activation, thrombosis and improve COVID-19 outcomes. Depending on the stage of exposure to and infection by SARS-CoV-2, enhancing adenosine levels by targeting key adenosine regulators such as ADA, ADK and equilibrative nucleoside transporter 1 might find therapeutic use against COVID-19 and warrants further investigation.
Related collections
Most cited references66
- Record: found
- Abstract: found
- Article: not found
Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China
- Record: found
- Abstract: found
- Article: not found
Clinical Characteristics of Coronavirus Disease 2019 in China
- Record: found
- Abstract: found
- Article: not found