- Record: found
- Abstract: found
- Article: found
Biting behaviour, spatio-temporal dynamics, and the insecticide resistance status of malaria vectors in different ecological zones in Ghana
Read this article at
Abstract
Background
A significant decrease in malaria morbidity and mortality has been attained using long-lasting insecticide-treated nets and indoor residual spraying. Selective pressure from these control methods influences changes in vector bionomics and behavioural pattern. There is a need to understand how insecticide resistance drives behavioural changes within vector species. This study aimed to determine the spatio-temporal dynamics and biting behaviour of malaria vectors in different ecological zones in Ghana in an era of high insecticide use for public health vector control.
Methods
Adult mosquitoes were collected during the dry and rainy seasons in 2017 and 2018 from five study sites in Ghana in different ecological zones. Indoor- and outdoor-biting mosquitoes were collected per hour from 18:00 to 06:00 h employing the human landing catch (HLC) technique. Morphological and molecular species identifications of vectors were done using identification keys and PCR respectively. Genotyping of insecticide-resistant markers was done using the TaqMan SNP genotyping probe-based assays. Detection of Plasmodium falciparum sporozoites was determined using PCR.
Results
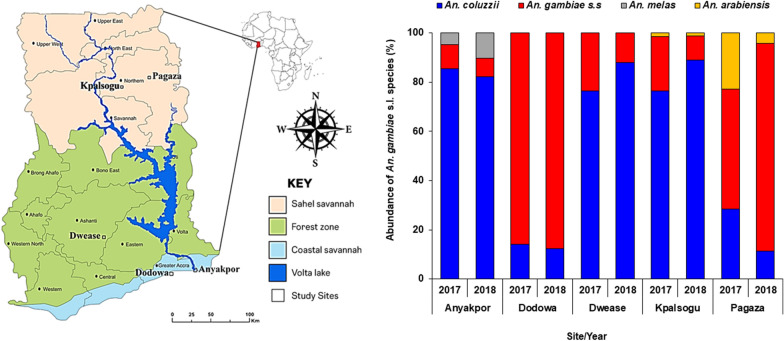
A total of 50,322 mosquitoes belonging to four different genera were collected from all the study sites during the sampling seasons in 2017 and 2018. Among the Anophelines were Anopheles gambiae s.l. 93.2%, (31,055/33,334), An. funestus 2.1%, (690/33,334), An. pharoensis 4.6%, (1545/33,334), and An. rufipes 0.1% (44/33,334). Overall, 76.4%, (25,468/33,334) of Anopheles mosquitoes were collected in the rainy season and 23.6%, (7866/33,334) in the dry season. There was a significant difference ( Z = 2.410; P = 0.0160) between indoor-biting (51.1%; 15,866/31,055) and outdoor-biting An. gambiae s.l. (48.9%; 15,189/31,055). The frequency of the Vgsc-1014F mutation was slightly higher in indoor-biting mosquitoes (54.9%) than outdoors (45.1%). Overall, 44 pools of samples were positive for P. falciparum CSP giving an overall sporozoite rate of 0.1%.
Conclusion
Anopheles gambiae s.l. were more abundant indoors across all ecological zones of Ghana. The frequency of G119S was higher indoors than outdoors from all the study sites, but with higher sporozoite rates in outdoor mosquitoes in Dodowa and Kpalsogu. There is, therefore, an urgent need for a supplementary malaria control intervention to control outdoor-biting mosquitoes.
Related collections
Most cited references51
- Record: found
- Abstract: found
- Article: not found
The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015
- Record: found
- Abstract: found
- Article: not found
Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction.
- Record: found
- Abstract: found
- Article: not found