- Record: found
- Abstract: found
- Article: found
Correction: Wnt11 Is Required for Oriented Migration of Dermogenic Progenitor Cells from the Dorsomedial Lip of the Avian Dermomyotome
correction
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
The figures and captions are in the incorrect order for Figs 1–8. Please see the corrected
figures here. The publisher apologizes for these errors.
10.1371/journal.pone.0203913.g001
Fig 1
Murine dermis volume and hair follicles number comparison between Wnt11 knock-out
and wild-type mice.
The diagram represents a graphical comparison of two averages with standard deviation
bars of mice skin volume in sections of E18.5 mice wild-type and Wnt11 knock-out mice.
The results showed that the dermis volume in the sections of Wnt11 knock-out mice
was approximately 45% lesser when compared to the wild-type mice sections. The graphical
comparison was performed in 20 sections after HE staining, 10 slides representing
3 mice for each genotype. The photos of the analysed sections were acquired at 40x
magnification. For the graph representing the hair follicles number, the hair placodes
on the back skin of the hybridized embryos for mDermo-1 were counted in identical
squares drawn on photos obtained using standardized magnification parameters. For
this experiment, 6 Wnt11 knock-out and 6 wild-type embryos were used. The diagram
representing the graphical comparison of the two averages with standard deviation
bars of hair follicles placodes number in mice embryos E14.5 hybridized for mDermo-1,
shows a decrease of 35% in the number of hair follicles placodes in the mutant mice.
10.1371/journal.pone.0203913.g002
Fig 2
Wnt11 expression in the DML is important for recruitment of dorsal dermal progenitors.
A. EGFP-transfected DML of chicken embryo at stage HH18-19, 20 hours after electroporation.
B. The embryo in A after 3 days of reincubation following electroporation. Note the
presence of EGFP positive cells in the myotome and region of the future dorsal dermis
(dotted circles). The white dotted lines squares delineate the somites. C. Cross-section
of the embryo in B. EGFP-positive cells can be seen to be migrating into the subectodermal
space overlying the spinal cord (white arrows). D. Wnt11 RNAi on chicken embryo. DML
of EFGP-Wnt11 RNAi construct transfected chicken embryos at HH18-19, 20 hours after
electroporation. E. After 3 days of reincubation following electroporation, the EGFP-Wnt11
RNAi expressing cells are only to be found in the myotome in a disorganized manner,
whereas EGFP-Wnt11 RNAi positive cells are missing in the dorsal dermis anlage. F.
In cross-section of the embryo in E, only very few cells (nearly undetectable) migrating
from DML are present into the subectodermal space (white arrow) overlying the spinal
cord (white line). White lines were traced along the neural tube for a better orientation.
G. Targeting of the DML at stage HH14-17 by EGFP-Wnt11 RNAi constructs after 24 hours
reincubation and the corresponding Wnt11 silencing as seen by ISH (area between black
arrows in H). I. There is no evidence of increased cell death at the sites of EGFP-Wnt11
construct transfection as seen by TUNEL staining. J. TUNEL staining of an electroporated
embryo with scrambled DNA was used as control for our TUNEL assay. K. Represents the
untreated control (Control 2) for TUNEL assay. Photos in I, J, K show the DML of the
coressponding sections after TUNEL assay. Black arrows in I, J, K point towards Tunel-positive
cells. NT: neural tube. DML: dorso-medial lip. Scale bar: 100 μm.
10.1371/journal.pone.0203913.g003
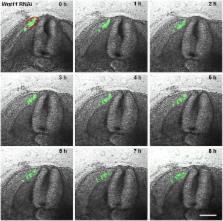
Fig 3
A series of 9 photos of a chicken embryo section electroporated with a control EGFP
plasmid at the DML level and reincubated for 24 h.
Each photo shows movement of cells in 1h time interval. At 0h the EGFP transfected
cells are seen in the DML. At later time points, the dynamics of the transfected cells
are visible, with cells moving to the subectodermal space above the neural tube (black
arrows), and additionally migrating to the immediate neighbourhood of the neural tube
(white arrows) and the myotome. These dynamic movements are characteristic of a normal
distribution of DML cells, where DML cells migrate to the subectodermal space in order
to form the dorsal dermis and to the myotome to form the muscle. The red outline indicates
the DML. Scale bar: 100 μm.
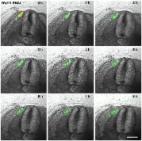
10.1371/journal.pone.0203913.g004
Fig 4
A series of 9 photos of a chicken embryo section electroporated with a Wnt11 RNAi
construct containing EGFP at the DML level, and then reincubated for 24h.
Each photo shows movement of cells in 1h time interval. In contrast to the control
experiment (Fig 2), the electroporated cells (green fluorescent) remain restricted
to the DML or migrate into the myotome. No EGFP positive cells migrate towards the
subectodermal space located above the neural tube or into the immediate neigbourhood.
The absence of Wnt11 in the DML thus results in a compromised EMT of the cells, which
can only enter the myotome and no longer populate the subectodermal space above the
neural tube. The red outline indicates the DML. Scale bar: 100 μm.
10.1371/journal.pone.0203913.g005
Fig 5
Decrease of dermal markers after Wnt11 RNAi.
A. Embryo showing the region of transfection after electroporation with EGFP-Wnt11
RNAi. The embryo has been electroporated at stage HH14-17 and reincubated for 20 hours.
B. The embryo in A after 2.5 days reincubation time following manipulation (HH28).
Starting with stage 24, cDermo-1 expression pattern can be found in the subectodermal
mesenchyme of the trunk, and at stage 26 it is very strong along the dorsal midline.
Following additional 2.5 days of reincubation after EGFP signal documentation for
embryo in A, cDermo-1 is remarkably reduced along the dorsal midline in the transfected
region (area between white arrows in B). C. Higher magnification of the embryo in
B. D. Electroporated embryo with Wnt11 silencing construct at stage HH14-17 and photographed
20 hours later, at stage HH20. E. After longer reincubation periods following electroporation
(4.5 days of the embryo in D), the embryo shows a retarded feather bud development
as seen after cDermo-1 ISH, which is expressed in this stage (HH31) in the mesenchyme
of the nascent feather buds (the white arrowheads in E and F mark the missing row
of feather buds on the manipulated side in D). F. Higher magnification of the embryo
in E. G. Embryo showing the region of transfection after electroporation with EGFP-Wnt11
RNAi at stage HH14-17 and photographed 24 hours later, at stage HH20. H. After 8 days
reincubation for embryo in G after EGFP documentation (HH38) and hybridisation with
a Shh probe we have noticed a retarded feather bud development as seen with cDermo-1.
In situ hybridisation for Shh also reveals a changed morphology of the eventually
formed feather buds, which are smaller and more flattened, with lower expression of
Shh on the manipulated side (white arrow and arrowheads in H). The white square presents
in a higher magnification the altered feather buds formation (yellow arrow). Scale
bar: 100 μm.
10.1371/journal.pone.0203913.g006
Fig 6
Effects of Wnt11 silencing on dermomyotome.
A, C, E, G and I. Chicken embryos electroporated with Wnt11 RNAi at stage HH14-17
and after a reincubation period of 24 hours. B. The embryo in A (HH20) hybridized
for En-1, shows that the central dermomyotomal compartment marked with En-1 was unaffected
in the electroporated area (the space between white arrows in B). D. The photo represents
the embryo in C after ISH for Sim-1 probe. The lateral dermomyotomal compartment marked
with Sim-1 (white arrows in D indicate the electroporated area) did not show any change
in its expression after Wnt11 silencing. F. ISH for Paraxis of the embryo presented
in photo E. Paraxis transcripts seems not to be altered following Wnt11 silencing
in the DML (area between white arrows in F). H. The dermomyotomal marker Pax3, in
contrast, is significantly upregulated at the site of Wnt11 RNAi transfection (area
between white arrows in H). K. The cross-section through the embryo in H in the manipulated
area shows a strong upregulation of Pax3 in the DML, while the DM remains normal when
compared to the control side. I. Electroporated embryo at stage HH14-17 with Wnt11
RNAi and after 24 hours reincubation (HH19). J. Hybridized embryo from photo I for
Snail1 probe. At stage HH20 the EMT has already started, and the Snail1 expression
can be seen in the myotome, dermomyotome, sclerotome and in the space above the neural
tube (dermal progenitor cells). Whole-mount ISH of the embryo electroporated with
Wnt11 RNAi shows a decreased Snail1 expression (the region in the bracket), while
the white arrows point towards the normal expression of Snail1 above the neural tube
(dermogenic progenitors) at untreated levels. L. Section through the embryo in J in
the affected region shows a decreased Snail1 expression in the DML (black arrowhead)
and above the neural tube (black arrow). Scale bar: 100 μm.
10.1371/journal.pone.0203913.g007
Fig 7
BMP2 signaling controls Wnt11 expression in DML.
A, B. BMP2 beads implanted into somites of chicken embryos (black arrow in A) lead
to an upregulation of Wnt11 as seen by ISH in vibratome sections (black arrow in B;
section level indicated by red line in A). C, D. In contrast, grafting of Noggin cells
(BMP antagonist) lead to a downregulation of Wnt11 transcripts on the operated side
(C) also visible in vibratome sections of the same embryo (black arrow in D; section
level indicated by red line in C). Scale bar: 100 μm.
10.1371/journal.pone.0203913.g008
Fig 8
Dorsal dermis is decreased in dermis thickness and number of hair follicles in the
Wnt11 knock-out mice.
A, C, E. Homozygous Wnt11 knock-out embryos. B, D, F. Wild-type embryos. Embryo in
A represents a homozygous Wnt11 knock-out embryo of embryonic day E 14.5. In the dorsal
mid-line region of this embryo a large area devoid of hair placodes is detectable.
The white square shows a decrease number of hair placodes in comparison to the WT
embryo of similar developmental stage (B). Section through the embryo in A shows that
the cDermo-1 signal is diminished with corresponding decrease in dermal thickness
in the mutant (black arrow in C) as compared with a similar section of the same magnification
of a WT embryo (black arrow in D). Hematoxylin-Eosin staining in paraffin sections
of knock-out mice of E 18.5 shows a remarkable decrease in dermis thickness (45%),
as well as a reduced number of hair follicles (35%). (E) In comparison with a Hematoxylin-Eosin
treated section of a WT mouse of the same developmental stage (F). Double-headed arrows
in E and F show the remarkable difference in dermis thickness between the mutant mouse
embryo as compared to the normal thickness of dermis in a wild-type embryo (photographs
were taken at the same magnification). The sections were taken at the trunk regions
of the mice. The analysis was restricted to the same anatomical area of the dorsal
skin for all embryos investigated. Scale bar: 100 μm.
Related collections
Most cited references1
- Record: found
- Abstract: found
- Article: found