- Record: found
- Abstract: found
- Article: found
Effect of Methylphenidate in Patients with Cancer-Related Fatigue: A Systematic Review and Meta-Analysis
Read this article at
Abstract
Background
Cancer-related fatigue (CRF) is a common symptom affecting patients with cancer. There are an increasing number of trials examining potential treatments for CRF. Methylphenidate represents one of the most researched drugs and an up-to-date assessment of the evidence for its use is needed. Trials of methylphenidate for CRF provided inconsistent results. This meta-analysis was aimed at assessing the effect and safety of methylphenidate on CRF.
Methods
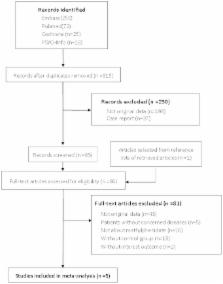
We comprehensively searched the Pubmed, EMBASE, PSYCHInfo and the Cochrane databases in order to identify published studies on the effect of methylphenidate on CRF. Primary outcomes included fatigue. Secondary outcomes included depression, cognition and adverse effects.
Findings
A meta-analysis was conducted on five randomized controlled trials and 498 patients were enrolled. Despite a large placebo effect observed in the studies included, pooled data suggested therapeutic effect of methylphenidate on CRF. Subgroup Analyses showed that the efficacy of methylphenidate on CRF is getting better with prolonging treatment duration, with a MD of −3.70 (95% CI −7.03– −0.37, p = 0.03) for long-time group and a MD of −2.49 (95% CI −6.01–1.03, p = 0.17) for short-time group. In general, there was no impact of methylphenidate on depression and cognition associated with CRF. Adverse events were similar between methylphenidate and placebo groups except that more patients reported vertigo, anxiety, anorexia and nausea in methylphenidate group compared to placebo group.
Related collections
Most cited references28
- Record: found
- Abstract: found
- Article: not found
The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory.
- Record: found
- Abstract: found
- Article: not found
Evidence report on the occurrence, assessment, and treatment of fatigue in cancer patients.
- Record: found
- Abstract: found
- Article: not found