- Record: found
- Abstract: found
- Article: found
Differential Expression Patterns of Rspondin Family and Leucine-Rich Repeat-Containing G-Protein Coupled Receptors in Chondrocytes and Osteoblasts
Read this article at
Abstract
Objective
Rspondins (RSPOs) are regarded as the significant modulators of WNT signaling pathway and they are expressed dynamically during developmental stages. Since in osteoarthritis (OA) both cartilage and subchondral bone suffer damages and WNT signaling pathway has a crucial role in their maintenance, the objective of the study was to analyze expression profile of RSPO family and its receptors [leucine-rich repeat-containing G-protein coupled receptors (LGRs)] in OA tissue samples as well as in differentiating chondrocytes and osteoblasts.
Materials and Methods:
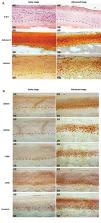
In this experimental study, human early and advanced stage of OA tissue samples were analyzed for the morphological changes of articular cartilage by hematoxylin and eosin (H&E) staining, safranin-O staining and lubricin immunostaining. RSPOs and LGRs expression were confirmed by immunohistochemistry. Human primary chondrocytes and human osteoblast cell line, SaOS-2, were cultured in differentiation medium till day 14 and they were analyzed in terms of expression of RSPOs, LGRs and specific marker for chondrogenesis and osteogenesis by western blotting and quantitative reverse transcription polymerase chain reaction (qRT-PCR).
Results
Advanced stage OA tissue samples showed increased expression of RSPO1 and LGR6 in a region close to subchondral bone. While RSPO2 and LGR5 expression were seen overlapping in the deep region of articular cartilage. Differentiating chondrocytes demonstrated elevated expression of RSPO2 and LGR5 from day 7 to day 14, whereas, osteoblasts undergoing differentiation showed enhanced expression of RSPO1 and LGR6 from day 2 to day 14. Under tumor necrosis factor alpha (TNFα) stimulatory conditions, RSPO2 and RSPO1 recovered the suppressed expression of inflammatory, chondrogenic and osteogenic markers, respectively. A recovery in the stability of β-catenin was also noticed in both cases.
Related collections
Most cited references40
- Record: found
- Abstract: found
- Article: not found
The bone-cartilage unit in osteoarthritis.
- Record: found
- Abstract: found
- Article: not found
Characterization of articular cartilage and subchondral bone changes in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis.
- Record: found
- Abstract: found
- Article: not found