- Record: found
- Abstract: found
- Article: found
Biomolecular environment, quantification, and intracellular interaction of multifunctional magnetic SERS nanoprobes†
Read this article at
Abstract
Abstract
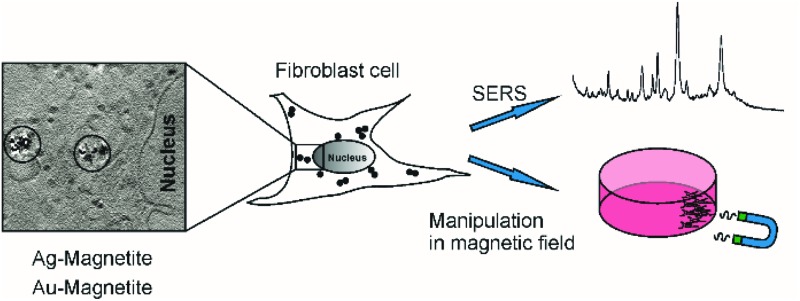
Multifunctional composite nanoprobes consisting of iron oxide nanoparticles linked to silver and gold nanoparticles, Ag–Magnetite and Au–Magnetite, respectively, were introduced by endocytic uptake into cultured fibroblast cells. The cells containing the non-toxic nanoprobes were shown to be displaceable in an external magnetic field and can be manipulated in microfluidic channels. The distribution of the composite nanostructures that are contained in the endosomal system is discussed on the basis of surface-enhanced Raman scattering (SERS) mapping, quantitative laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) micromapping, and cryo soft X-ray tomography (cryo soft-XRT). Cryo soft-XRT of intact, vitrified cells reveals that the composite nanoprobes form intra-endosomal aggregates. The nanoprobes provide SERS signals from the biomolecular composition of their surface in the endosomal environment. The SERS data indicate the high stability of the nanoprobes and of their plasmonic properties in the harsh environment of endosomes and lysosomes. The spectra point at the molecular composition at the surface of the Ag–Magnetite and Au–Magnetite nanostructures that is very similar to that of other composite structures, but different from the composition of pure silver and gold SERS nanoprobes used for intracellular investigations. As shown by the LA-ICP-MS data, the uptake efficiency of the magnetite composites is approximately two to three times higher than that of the pure gold and silver nanoparticles.
Related collections
Most cited references71
- Record: found
- Abstract: found
- Article: not found
Nuclear pore complex is able to transport macromolecules with diameters of about 39 nm.
- Record: found
- Abstract: found
- Article: not found
Continuous sorting of magnetic cells via on-chip free-flow magnetophoresis.
- Record: found
- Abstract: found
- Article: not found
Combined microfluidic-micromagnetic separation of living cells in continuous flow.
Author and article information
Notes
†Electronic supplementary information (ESI) available. See DOI: 10.1039/c6an00890a