- Record: found
- Abstract: found
- Article: found
GPCRs Direct Germline Development and Somatic Gonad Function in Planarians
Read this article at
Abstract
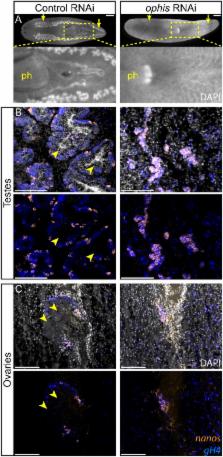
Planarians display remarkable plasticity in maintenance of their germline, with the ability to develop or dismantle reproductive tissues in response to systemic and environmental cues. Here, we investigated the role of G protein-coupled receptors (GPCRs) in this dynamic germline regulation. By genome-enabled receptor mining, we identified 566 putative planarian GPCRs and classified them into conserved and phylum-specific subfamilies. We performed a functional screen to identify NPYR-1 as the cognate receptor for NPY-8, a neuropeptide required for sexual maturation and germ cell differentiation. Similar to NPY-8, knockdown of this receptor results in loss of differentiated germ cells and sexual maturity. NPYR-1 is expressed in neuroendocrine cells of the central nervous system and can be activated specifically by NPY-8 in cell-based assays. Additionally, we screened the complement of GPCRs with expression enriched in sexually reproducing planarians, and identified an orphan chemoreceptor family member, ophis, that controls differentiation of germline stem cells (GSCs). ophis is expressed in somatic cells of male and female gonads, as well as in accessory reproductive tissues. We have previously shown that somatic gonadal cells are required for male GSC specification and maintenance in planarians. However, ophis is not essential for GSC specification or maintenance and, therefore, defines a secondary role for planarian gonadal niche cells in promoting GSC differentiation. Our studies uncover the complement of planarian GPCRs and reveal previously unappreciated roles for these receptors in systemic and local (i.e., niche) regulation of germ cell development.
Abstract
Genome-wide analysis of the planarian Schmidtea mediterranea reveals a complement of over 550 G protein-coupled receptors, including two with critical roles in germline development.
Author Summary
G protein-coupled receptors (GPCRs) are the largest and most versatile family of cell-surface receptors. They play critical roles in various cellular and physiological systems and have emerged as a leading group of therapeutic targets. Due to their structural and functional conservation across animals, much has been learned about GPCRs from studies in laboratory models. Here, we performed genome-wide receptor mining to identify and categorize the complement of GPCR-encoding genes in the planarian Schmidtea mediterranea, an emerging model organism for regeneration and germ cell biology. We then conducted two studies implicating planarian GPCRs in the regulation of reproductive function. First, we found the receptor component of a central neuropeptide Y signaling pathway and demonstrated its involvement in the systemic control of reproductive development. Next, we showed that a novel chemoreceptor family member is expressed in somatic cells of the planarian gonads and directs germ cell maturation via the niche. We predict that future studies on the hundreds of other planarian GPCRs identified in this work will not only help us understand the conserved role of these receptors in various physiological pathways but also pave the way for identification of novel therapeutic targets in parasitic relatives of the planarian.
Related collections
Most cited references70
- Record: found
- Abstract: found
- Article: not found
The evolution of gene expression levels in mammalian organs.
- Record: found
- Abstract: found
- Article: not found
Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54.
- Record: found
- Abstract: found
- Article: not found