- Record: found
- Abstract: found
- Article: found
A new FRDA mouse model [ Fxn null:YG8s(GAA) > 800] with more than 800 GAA repeats
Read this article at
Abstract
Introduction
Friedreich’s ataxia (FRDA) is an inherited recessive neurodegenerative disorder caused by a homozygous guanine-adenine-adenine (GAA) repeat expansion within intron 1 of the FXN gene, which encodes the essential mitochondrial protein frataxin. There is still no effective therapy for FRDA, therefore the development of optimal cell and animal models of the disease is one of the priorities for preclinical therapeutic testing.
Methods
We obtained the latest FRDA humanized mouse model that was generated on the basis of our previous YG8sR, by Jackson laboratory [YG8JR, Fxn null:YG8s(GAA) > 800]. We characterized the behavioral, cellular, molecular and epigenetics properties of the YG8JR model, which has the largest GAA repeat sizes compared to all the current FRDA mouse models.
Results
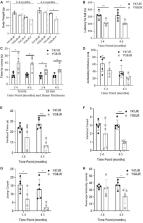
We found statistically significant behavioral deficits, together with reduced levels of frataxin mRNA and protein, and aconitase activity in YG8JR mice compared with control Y47JR mice. YG8JR mice exhibit intergenerational GAA repeat instability by the analysis of parent and offspring tissue samples. Somatic GAA repeat instability was also detected in individual brain and cerebellum tissue samples. In addition, increased DNA methylation of CpG U13 was identified in FXN GAA repeat region in the brain, cerebellum, and heart tissues. Furthermore, we show decreased histone H3K9 acetylation and increased H3K9 methylation of YG8JR cerebellum tissues within the FXN gene, upstream and downstream of the GAA repeat region compared to Y47JR controls.
Related collections
Most cited references65
- Record: found
- Abstract: found
- Article: not found
Friedreich's ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion.
- Record: found
- Abstract: found
- Article: not found
Aconitase and mitochondrial iron-sulphur protein deficiency in Friedreich ataxia.
- Record: found
- Abstract: found
- Article: not found