- Record: found
- Abstract: found
- Article: found
Identification of a small molecule that primes the type I interferon response to cytosolic DNA
Read this article at
Abstract
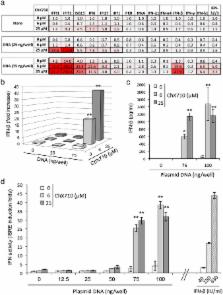
The type I interferon response plays a pivotal role in host defense against infectious agents and tumors, and promising therapeutic approaches rely on small molecules designed to boost this system. To identify such compounds, we developed a high-throughput screening assay based on HEK-293 cells expressing luciferase under the control of Interferon-Stimulated Response Elements (ISRE). An original library of 10,000 synthetic compounds was screened, and we identified a series of 1H-benzimidazole-4-carboxamide compounds inducing the ISRE promoter sequence, specific cellular Interferon-Stimulated Genes (ISGs), and the phosphorylation of Interferon Regulatory Factor (IRF) 3. ISRE induction by ChX710, a prototypical member of this chemical series, was dependent on the adaptor MAVS and IRF1, but was IRF3 independent. Although it was unable to trigger type I IFN secretion per se, ChX710 efficiently primed cellular response to transfected plasmid DNA as assessed by potent synergistic effects on IFN-β secretion and ISG expression levels. This cellular response was dependent on STING, a key adaptor involved in the sensing of cytosolic DNA and immune activation by various pathogens, stress signals and tumorigenesis. Our results demonstrate that cellular response to cytosolic DNA can be boosted with a small molecule, and potential applications in antimicrobial and cancer therapies are discussed.
Related collections
Most cited references37
- Record: found
- Abstract: found
- Article: not found
Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides.
- Record: found
- Abstract: found
- Article: not found
DNA damage primes the type I interferon system via the cytosolic DNA sensor STING to promote anti-microbial innate immunity.
- Record: found
- Abstract: found
- Article: not found