- Record: found
- Abstract: found
- Article: found
Interleukin-16 is increased in obesity and alters adipogenesis and inflammation in vitro
Read this article at
Abstract
Introduction
Obesity is a chronic condition associated with low-grade inflammation mainly due to immune cell infiltration of white adipose tissue (WAT). WAT is distributed into two main depots: subcutaneous WAT (sWAT) and visceral WAT (vWAT), each with different biochemical features and metabolic roles. Proinflammatory cytokines including interleukin (IL)-16 are secreted by both adipocytes and infiltrated immune cells to upregulate inflammation. IL-16 has been widely studied in the peripheral proinflammatory immune response; however, little is known about its role in adipocytes in the context of obesity.
Aim & Methods
We aimed to study the levels of IL-16 in WAT derived from sWAT and vWAT depots of humans with obesity and the role of this cytokine in palmitate-exposed 3T3-L1 adipocytes.
Results
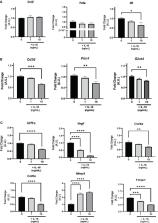
The results demonstrated that IL-16 expression was higher in vWAT compared with sWAT in individuals with obesity. In addition, IL-16 serum levels were higher in patients with obesity compared with normal-weight individuals, increased at 6 months after bariatric surgery, and at 12 months after surgery decreased to levels similar to before the intervention. Our in vitro models showed that IL-16 could modulate markers of adipogenesis (Pref1), lipid metabolism (Plin1, Cd36, and Glut4), fibrosis (Hif1a, Col4a, Col6a, and Vegf), and inflammatory signaling (IL6) during adipogenesis and in mature adipocytes. In addition, lipid accumulation and glycerol release assays suggested lipolysis alteration.
Related collections
Most cited references58
- Record: found
- Abstract: found
- Article: not found
Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study.
- Record: found
- Abstract: found
- Article: not found
Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies
- Record: found
- Abstract: found
- Article: not found