- Record: found
- Abstract: found
- Article: found
Gene expression profiles in the bovine corpus luteum (CL) during the estrous cycle and pregnancy: Possible roles of chemokines in regulating CL function during pregnancy
Read this article at
Abstract
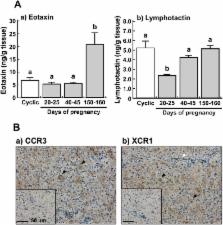
To determine functional differences between the corpus luteum (CL) of the estrous cycle and pregnancy in cows, gene expression profiles were compared using a 15 K bovine oligo DNA microarray. In the pregnant CL at days 20–25, 40–45 and 150–160, the expressions of 138, 265 and 455 genes differed by a factor of > 2-fold (P < 0.05) from their expressions in the cyclic CL (days 10–12 of the estrous cycle). Messenger RNA expressions of chemokines ( eotaxin, lymphotactin and ENA-78) and their receptors ( CCR3, XCR1 and CXCR2) were validated by quantitative real-time PCR. Transcripts of eotaxin were more abundant in the CL at days 40–45 and 150–160 of pregnancy than in the cyclic CL (P < 0.01). In contrast, the mRNA expressions of lymphotactin, ENA-78 and XCR1 were lower in the CL of pregnancy (P < 0.05). Messenger RNAs of CCR3 and CXCR2 were similarly detected both in the cyclic and pregnant CL. Tissue protein levels of eotaxin were significantly higher in the CL at days 150–160 of pregnancy than in the CL at other stages, whereas the lymphotactin protein levels in the CL at days 20–25 of pregnancy were lower (P < 0.05). Immunohistochemical staining showed that CCR3 was expressed in the luteal cells and that XCR1 was expressed in both the luteal cells and endothelial cells. Collectively, the different gene expression profiles may contribute to functional differences between the cyclic and pregnant CL, and chemokines including eotaxin and lymphotactin may regulate CL function during pregnancy in cows.
Related collections
Most cited references38
- Record: found
- Abstract: found
- Article: not found
Lymphocyte traffic control by chemokines.
- Record: found
- Abstract: found
- Article: not found
Mechanisms controlling the function and life span of the corpus luteum.
- Record: found
- Abstract: found
- Article: not found