- Record: found
- Abstract: found
- Article: found
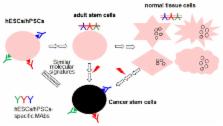
Cancer stem cell surface markers on normal stem cells
Read this article at
Abstract
The cancer stem cell (CSC) hypothesis has captured the attention of many scientists. It is believed that elimination of CSCs could possibly eradicate the whole cancer. CSC surface markers provide molecular targeted therapies for various cancers, using therapeutic antibodies specific for the CSC surface markers. Various CSC surface markers have been identified and published. Interestingly, most of the markers used to identify CSCs are derived from surface markers present on human embryonic stem cells (hESCs) or adult stem cells. In this review, we classify the currently known 40 CSC surface markers into 3 different categories, in terms of their expression in hESCs, adult stem cells, and normal tissue cells. Approximately 73% of current CSC surface markers appear to be present on embryonic or adult stem cells, and they are rarely expressed on normal tissue cells. The remaining CSC surface markers are considerably expressed even in normal tissue cells, and some of them have been extensively validated as CSC surface markers by various research groups. We discuss the significance of the categorized CSC surface markers, and provide insight into why surface markers on hESCs are an attractive source to find novel surface markers on CSCs.
Related collections
Most cited references118
- Record: found
- Abstract: found
- Article: not found
Identification of pancreatic cancer stem cells.
- Record: found
- Abstract: found
- Article: not found
Identification of cells initiating human melanomas.
- Record: found
- Abstract: found
- Article: not found