- Record: found
- Abstract: found
- Article: not found
Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: a current perspective

Graphical abstract
Abstract
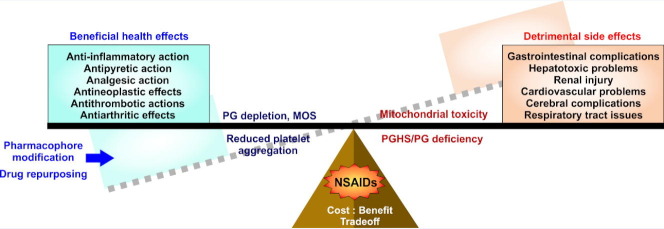
Owing to the efficacy in reducing pain and inflammation non-steroidal anti-inflammatory drugs (NSAIDs) are amongst the most popularly used medicines, confirming their position in the WHO’s Model List of Essential Medicines. With escalating musculoskeletal complications, as evident from 2016 Global Burden of Disease data, NSAID usage is evidently unavoidable. Apart from analgesic, anti-inflammatory and antipyretic efficacies, NSAIDs are further documented to offer protection against diverse critical disorders including cancer and heart attacks. However, data from multiple placebo-controlled trials and meta-analyses studies alarmingly signify the adverse effects of NSAIDs in gastrointestinal, cardiovascular, hepatic, renal, cerebral and pulmonary complications. Although extensive research has elucidated the mechanisms underlying the clinical hazards of NSAIDs, no review has extensively collated the outcomes on various multiorgan toxicities of these drugs together. In this regard, the present review provides a comprehensive insight of the existing knowledge and recent developments on NSAID-induced organ damage. It precisely encompasses the current understanding of structure, classification and mode of action of NSAIDs while reiterating on the emerging instances of NSAID drug repurposing along with pharmacophore modification aimed at safer usage of NSAIDs where toxic effects are tamed without compromising the clinical benefits. The review does not intend to vilify these ‘wonder drugs’; rather provide a careful understanding of their side-effects which would be beneficial in evaluating the risk-benefit threshold while rationally using NSAIDs at safer dose and duration.
Related collections
Most cited references203
- Record: found
- Abstract: not found
- Article: not found
Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs.
- Record: found
- Abstract: found
- Article: not found
Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group.
- Record: found
- Abstract: found
- Article: not found