- Record: found
- Abstract: found
- Article: found
Protein Delivery of Cell-Penetrating Zinc-Finger Activators Stimulates Latent HIV-1-Infected Cells

Read this article at
Abstract
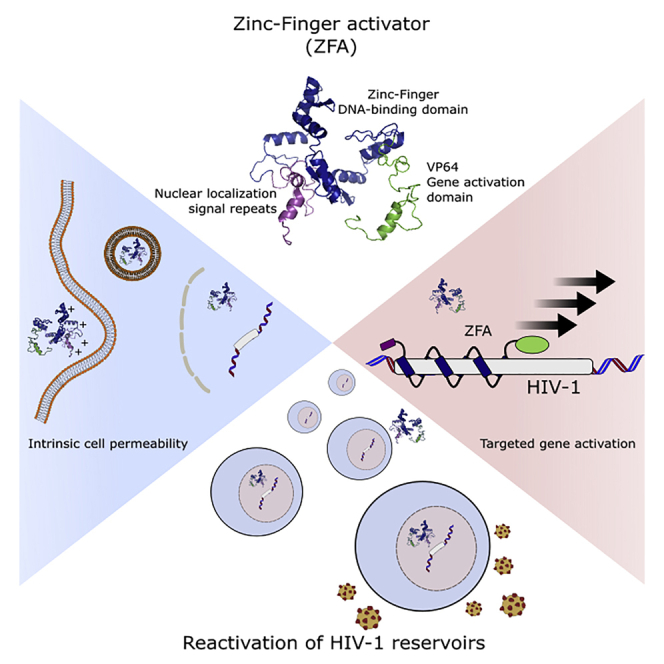
Despite efforts to develop effective treatments for eradicating HIV-1, a cure has not yet been achieved. Whereas antiretroviral drugs target an actively replicating virus, latent, nonreplicative forms persist during treatment. Pharmacological strategies that reactivate latent HIV-1 and expose cellular reservoirs to antiretroviral therapy and the host immune system have, so far, been unsuccessful, often triggering severe side effects, mainly due to systemic immune activation. Here, we present an alternative approach for stimulating latent HIV-1 expression via direct protein delivery of cell-penetrating zinc-finger activators (ZFAs). Cys 2-His 2 zinc-fingers, fused to a transcription activation domain, were engineered to recognize the HIV-1 promoter and induce targeted viral transcription. Following conjugation with multiple positively charged nuclear localization signal (NLS) repeats, protein delivery of a single ZFA (3NLS-PBS1-VP64) efficiently internalized HIV-1 latently infected T-lymphocytes and specifically stimulated viral expression. We show that short-term treatment with this ZFA protein induces higher levels of viral reactivation in cell line models of HIV-1 latency than those observed with gene delivery. Our work establishes protein delivery of ZFA as a novel and safe approach toward eradication of HIV-1 reservoirs.
Graphical Abstract
Abstract
Eradication of HIV-1 infection is impeded by the persistence of cellular reservoirs harboring latent provirus. Stimulation of latent viral expression is considered critical to target HIV reservoirs for elimination. Perdigão et al. established a “gene-free” approach to specifically activate latent HIV expression through protein delivery of cell-penetrating zinc-finger activators. Engineered zinc-finger activators can translocate across the cell membrane and target the HIV promoter to induce viral expression from latently infected cells, providing a novel and safe route for the elimination of HIV-1 reservoirs.
Related collections
Most cited references64
- Record: found
- Abstract: found
- Article: not found
HIV reproducibly establishes a latent infection after acute infection of T cells in vitro.
- Record: found
- Abstract: found
- Article: not found
Delivery technologies for genome editing
- Record: found
- Abstract: found
- Article: not found