- Record: found
- Abstract: found
- Article: found
Trajectories of oral bisphosphonate use after hip fractures: a population-based cohort study
Read this article at
Abstract
Summary
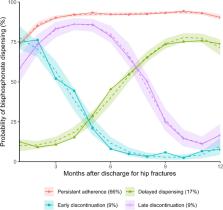
Bisphosphonates prevent future hip fractures. However, we found that one in six patients with hip fractures had a delay in bisphosphonate initiation and another one-sixth discontinued treatment within 12 months after discharge. Our results highlight the need to address hesitancy in treatment initiation and continuous monitoring.
Purpose
Suboptimal antiresorptive use is not well understood. This study investigated trajectories of oral bisphosphonate use following first hip fractures and factors associated with different adherence and persistence trajectories.
Methods
We conducted a retrospective study of all patients aged ≥ 50 years dispensed two or more bisphosphonate prescriptions following first hip fracture in Victoria, Australia, from 2012 to 2017. Twelve-month trajectories of bisphosphonate use were categorized using group-based trajectory modeling. Factors associated with different trajectories compared to the persistent adherence trajectory were assessed using multivariate multinomial logistic regression.
Results
We identified four patterns of oral bisphosphonate use in 1811 patients: persistent adherence (66%); delayed dispensing (17%); early discontinuation (9%); and late discontinuation (9%). Pre-admission bisphosphonate use was associated with a lower risk of delayed dispensing in both sexes (relative risk [RR] 0.28, 95% confidence interval [CI] 0.21–0.39). Older patients ( \documentclass[12pt]{minimal} \usepackage{amsmath} \usepackage{wasysym} \usepackage{amsfonts} \usepackage{amssymb} \usepackage{amsbsy} \usepackage{mathrsfs} \usepackage{upgreek} \setlength{\oddsidemargin}{-69pt} \begin{document}$$\ge$$\end{document} 85 years old versus 50–64 years old, RR 0.38, 95% CI 0.22–0.64) had a lower risk of delayed dispensing. Males with anxiety (RR 9.80, 95% CI 2.24–42.9) and females with previous falls had increased risk of early discontinuation (RR 1.80, 95% CI 1.16–2.78).
Related collections
Most cited references39

- Record: found
- Abstract: found
- Article: found
Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study
- Record: found
- Abstract: found
- Article: not found
Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration.
- Record: found
- Abstract: not found
- Article: not found