- Record: found
- Abstract: found
- Article: not found
Lessons to learn from tumor-educated platelets
Read this article at
Visual Abstract
Abstract
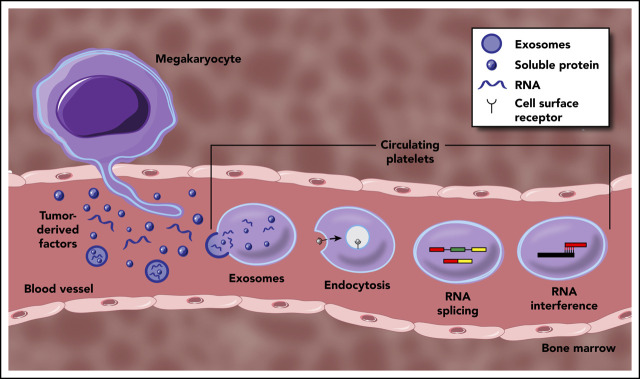
Platelets have long been known to play important roles beyond hemostasis and thrombosis. Now recognized as a bona fide mediator of malignant disease, platelets influence various aspects of cancer progression, most notably tumor cell metastasis. Interestingly, platelets isolated from cancer patients often display distinct RNA and protein profiles, with no clear alterations in hemostatic activity. This phenotypically distinct population, termed tumor-educated platelets, now receive significant attention for their potential use as a readily available liquid biopsy for early cancer detection. Although the mechanisms underpinning platelet education are still being defined, direct uptake and storage of tumor-derived factors, signal-dependent changes in platelet RNA processing, and differential platelet production by tumor-educated megakaryocytes are the most prominent scenarios. This article aims to cover the various modalities of platelet education by tumors, in addition to assessing their diagnostic potential.
Related collections
Most cited references86
- Record: found
- Abstract: found
- Article: found
Intratumor Heterogeneity and Branched Evolution Revealed by Multiregion Sequencing
- Record: found
- Abstract: found
- Article: not found
The lung is a site of platelet biogenesis and a reservoir for hematopoietic progenitors
- Record: found
- Abstract: not found
- Article: not found