- Record: found
- Abstract: found
- Article: found
A positive feedback loop of ARF6 activates ERK1/2 signaling pathway via DUSP6 silencing to promote pancreatic cancer progression : ARF6 regulates ERK1/2 pathway by inhibiting DUSP6
Read this article at
Abstract
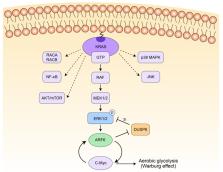
ERK1/2 are essential proteins mediating mitogen-activated protein kinase signaling downstream of RAS in pancreatic adenocarcinoma (PDAC). Our previous study reveals that ARF6 plays a positive regulatory role in ERK1/2 pathway in a feedback loop manner. A significant part of the literature on ARF6 has emphasized its oncogenic effect as an essential downstream molecule of ERK1/2, and no research has been done on the regulation mechanisms of the feedback loop between ARF6 and the ERK1/2 signaling pathway. In the present study, we explore the gene network downstream of ARF6 and find that DUSP6 may be the critical signal molecule in the positive feedback loop between ARF6 and ERK1/2. Specifically, to elucidate the negative correlations between ARF6 and DUSP6 in pancreatic cancer, we examine their expressions in pancreatic cancer tissues by immunohistochemical staining. Then the impact of DUSP6 on the proliferation and apoptosis of PDAC cells are investigated by gain-of-function and loss-of-function approaches. Mechanism explorations uncover that ARF6 suppresses the expression of DUSP6, which is responsible for the dephosphorylation of ERK1/2. Altogether, these results indicate that DUSP6 plays a tumor-suppressive role and acts as an intermediate molecule between ARF6 and ERK1/2 in PDAC cells, thereby forming a positive feedback loop.
Related collections
Most cited references30
- Record: found
- Abstract: found
- Article: not found
Cancer statistics, 2020
- Record: found
- Abstract: found
- Article: not found
Pancreatic cancer
- Record: found
- Abstract: found
- Article: not found