- Record: found
- Abstract: found
- Article: found
G i/o protein-coupled receptor inhibition of beta-cell electrical excitability and insulin secretion depends on Na +/K + ATPase activation
Read this article at
Abstract
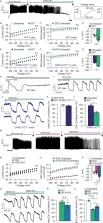
G i/o-coupled somatostatin or α2-adrenergic receptor activation stimulated β-cell NKA activity, resulting in islet Ca 2+ fluctuations. Furthermore, intra-islet paracrine activation of β-cell G i/o-GPCRs and NKAs by δ-cell somatostatin secretion slowed Ca 2+ oscillations, which decreased insulin secretion. β-cell membrane potential hyperpolarization resulting from G i/o-GPCR activation was dependent on NKA phosphorylation by Src tyrosine kinases. Whereas, β-cell NKA function was inhibited by cAMP-dependent PKA activity. These data reveal that NKA-mediated β-cell membrane potential hyperpolarization is the primary and conserved mechanism for G i/o-GPCR control of electrical excitability, Ca 2+ handling, and insulin secretion.
Abstract
G i/o protein-coupled receptors (G i/o-GPCRs) limit β-cell insulin secretion by decreasing Ca 2+ entry; however, the underlying mechanism has not been identified. Here, the authors show that G i/o-GPCRs hyperpolarize mouse and human β-cell membrane potential by activating Na +/K +ATPases.
Related collections
Most cited references94
- Record: found
- Abstract: found
- Article: not found
A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex.
- Record: found
- Abstract: found
- Article: not found
Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse.

- Record: found
- Abstract: found
- Article: found