- Record: found
- Abstract: found
- Article: found
HPV status and immunohistochemical analysis of p16, p53 and PD‑L1 expression as prognostic biomarkers in patients with squamous cell anal cancer receiving definitive radiotherapy/chemoradiotherapy
Read this article at
Abstract
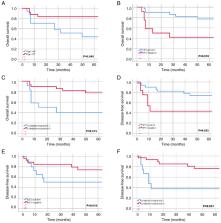
Anal squamous cell carcinoma (SCC) treated with definitive radiotherapy (RT)/chemoradiotherapy (CRT) has shown high success rates, yet challenges such as treatment resistance and recurrence persist. The present study aimed to investigate the associations between immunohistochemical (IHC) evaluation, treatment response and prognosis in anal SCC. A retrospective cohort analysis included 42 patients with anal SCC treated at a single institution between 2006 and 2022. Human papillomavirus (HPV) status was determined, and the IHC analysis of p16, p53 and PD-L1 expression was conducted using formalin-fixed, paraffin-embedded biopsies. A complete response to RT/CRT was observed in 71.4% of patients. Recurrence occurred in 38.1% of cases, of which 7.1% had local-regional recurrence (LRR), 14.3% had distant recurrence (DR), and 16.7% had both LRR and DR. HPV positivity (71.4%) was significantly associated with p16 positivity. Lack of complete response was associated with HPV-negative status, p16-negative status, increased recurrence and DR. In addition, recurrence was significantly associated with p53-positive status, and p53 positivity was significantly associated with increased LRR. PD-L1 positivity, defined as a combined positive score (CPS) ≥1% was found in 73.8% of the patients, and exhibited significant associations with HPV positivity and p16 positivity. PD-L1 CPS ≥ 1% was also associated with an increased LRR. Univariate analysis revealed that age <65 years, a complete response and HPV positivity were associated with increased 5-year overall survival (OS), while a complete response, HPV positivity and p53-negative status were associated with increased 5-year disease-free survival (DFS). Multivariate analysis identified that age <65 years and HPV positivity are independent prognostic factors for 5-year OS, and a complete response and p53-negative status are independent prognostic factors for 5-year DFS. In conclusion, these findings suggust that the identification of HPV status and poor prognostic biomarkers at diagnosis may be used to guide personalized treatment strategies, with the combination of immunotherapy with standard CRT potentially providing improved outcomes.
Related collections
Most cited references64
- Record: found
- Abstract: found
- Article: not found
New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1).
- Record: found
- Abstract: found
- Article: not found
Human papillomavirus and survival of patients with oropharyngeal cancer.
- Record: found
- Abstract: found
- Article: not found