- Record: found
- Abstract: found
- Article: not found
New developments in the genetics, pathogenesis, and therapy of IgA nephropathy
Read this article at
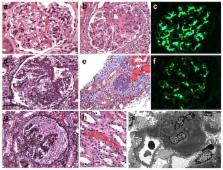
Abstract
Recent years have brought notable progress in the field of IgA nephropathy. Here, we highlight important new directions and latest developments, including successful discovery of several genetic susceptibility loci, formulation of the multi-hit pathogenesis model that integrates findings from studies of galactose-deficient IgA1, anti-glycan response and immune complex-induced kidney injury, introduction of the Oxford pathology scoring system, and formalization of the Kidney Disease Improving Global Outcomes (KDIGO) consensus treatment guidelines. We focus on the latest genetic findings that confirm a strong contribution of inherited factors and explain some of the geo-ethnic disparities in disease susceptibility. Most IgA nephropathy susceptibility loci discovered to date encode genes involved in the maintenance of the intestinal epithelial barrier and response to mucosal pathogens. The concerted pattern of inter-population allelic differentiation across all Genome Wide Association Studies (GWAS) loci parallels the disease prevalence and correlates with variation in local pathogens, suggesting that multi-locus adaptation might have shaped the present-day landscape of IgA nephropathy. Importantly, the “Intestinal Immune Network for IgA Production” emerged as one of the new targets for potential therapeutic intervention. We place these findings in the context of the multi-hit pathogenesis model and existing knowledge of IgA immunobiology. Lastly, we provide our perspective on the existing treatment options, discuss areas of clinical uncertainty, and outline ongoing clinical trials and translational studies.
Related collections
Most cited references153
- Record: found
- Abstract: found
- Article: not found
Transglutaminases: crosslinking enzymes with pleiotropic functions.
- Record: found
- Abstract: found
- Article: not found
Deep resequencing of GWAS loci identifies independent rare variants associated with inflammatory bowel disease
- Record: found
- Abstract: found
- Article: not found
