- Record: found
- Abstract: found
- Article: found
Efficacy and Safety of Lactobacillus Plantarum C29-Fermented Soybean (DW2009) in Individuals with Mild Cognitive Impairment: A 12-Week, Multi-Center, Randomized, Double-Blind, Placebo-Controlled Clinical Trial
Read this article at
Abstract
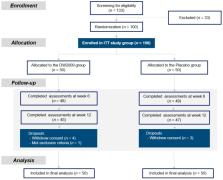
Early intervention using dietary supplements may be effective in alleviating cognitive impairment among individuals with mild cognitive impairment (MCI). This study investigated the efficacy and safety of Lactobacillus plantarum C29-fermented soybean (DW2009) as a nutritional supplement for cognitive enhancement. One hundred individuals with MCI were randomly assigned to take DW2009 (800 mg/day, n = 50) or placebo (800 mg/day, n = 50) for 12 weeks. The primary outcome measure was change in the composite score of cognitive functions related to memory and attention, measured by computerized neurocognitive function tests. Associations between changes in serum brain-derived neurotrophic factor (BDNF) levels and cognitive performance for each treatment group were evaluated. Compared to the placebo group, the DW2009 group showed greater improvements in the combined cognitive functions ( z = 2.36, p for interaction = 0.02), especially in the attention domain ( z = 2.34, p for interaction = 0.02). Cognitive improvement was associated with increased serum BDNF levels after consumption of DW2009 ( t = 2.83, p = 0.007). The results of this clinical trial suggest that DW2009 can be safely administered to enhance cognitive function in individuals with MCI. Increased serum BDNF levels after administering DW2009 may provide preliminary insight into the underlying effects of cognitive improvement, which suggests the importance of the gut-brain axis in ameliorating cognitive deficits in MCI.
Related collections
Most cited references26
- Record: found
- Abstract: not found
- Article: not found
The BDNF val66met Polymorphism Affects Activity-Dependent Secretion of BDNF and Human Memory and Hippocampal Function
- Record: found
- Abstract: found
- Article: not found
BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders.
- Record: found
- Abstract: found
- Article: not found