- Record: found
- Abstract: found
- Article: found
Regulatory mechanisms of the early phase of white adipocyte differentiation: an overview

Read this article at
Abstract
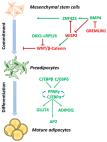
The adipose organ comprises two main fat depots termed white and brown adipose tissues. Adipogenesis is a process leading to newly differentiated adipocytes starting from precursor cells, which requires the contribution of many cellular activities at the genome, transcriptome, proteome, and metabolome levels. The adipogenic program is accomplished through two sequential phases; the first includes events favoring the commitment of adipose tissue stem cells/precursors to preadipocytes, while the second involves mechanisms that allow the achievement of full adipocyte differentiation. While there is a very large literature about the mechanisms involved in terminal adipogenesis, little is known about the first stage of this process. Growing interest in this field is due to the recent identification of adipose tissue precursors, which include a heterogenous cell population within different types of adipose tissue as well as within the same fat depot. In addition, the alteration of the heterogeneity of adipose tissue stem cells and of the mechanisms involved in their commitment have been linked to adipose tissue development defects and hence to the onset/progression of metabolic diseases, such as obesity. For this reason, the characterization of early adipogenic events is crucial to understand the etiology and the evolution of adipogenesis-related pathologies, and to explore the adipose tissue precursors’ potential as future tools for precision medicine.
Related collections
Most cited references119
- Record: found
- Abstract: found
- Article: not found
Adipogenesis and metabolic health
- Record: found
- Abstract: found
- Article: found
Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications

- Record: found
- Abstract: found
- Article: found