- Record: found
- Abstract: found
- Article: found
Autologous micro-fragmented adipose tissue injection provides significant and prolonged clinical improvement in patients with knee osteoarthritis: a case-series study
Read this article at
Abstract
Purpose
Among the conservative strategies to manage patients with symptomatic knee osteoarthritis (OA), an innovative approach exploiting the regenerative capability of adipose tissue and its resident MSCs (Mesenchymal Stem Cells or Medicinal Signalling Cells) has been proposed with encouraging results. This study aims to demonstrate the benefits of autologous micro-fragmented adipose tissue (MAT) injection in the conservative treatment of knee osteoarthritis and whether any variables may affect the outcome. This is a case series single-centre study in which patients underwent intraarticular MAT injection without any associated procedures.
Methods
Based on inclusion and exclusion criteria, 49 patients (67 Knees) were included and retrospectively analysed with a mean follow-up of 34.04 ± 13.62 months (minimum 11 – maximum 59). Patients were assessed through the WOMAC and KOOS questionnaires at baseline (pre-treatment) and 1-, 3-, 6-, 12-, 24- and 36-month follow-up. A minimal clinically important difference (MCID) of at least 7.5 points for the WOMAC pain scale and 7.2 for the WOMAC function scale compared to the baseline value was used.
Results
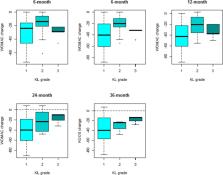
WOMAC and KOOS scores improved after treatment compared to baseline at all follow-ups with p < 0.001. Male gender and Kellgren-Lawrence (KL) grade 2 were associated with smaller improvement in WOMAC and KOOS scores (with respect to females and to KL grade 1, respectively) up to 24 months. The percentage of patients who reach the MCID for WOMAC pain is generally lower than that of patients who reach the MCID for WOMAC function (around 80% at all time points), but it increases significantly over time. Moreover, the baseline score of the WOMAC pain and function influence the outcome. Patients with worse symptoms are more likely to reach the MCID.
Conclusions
Intra-articular knee injection of MAT for the treatment of knee osteoarthritis (KOA), recalcitrant to traditional conservative treatments, proved to be effective in a high percentage of cases. The positive association between a worse pre-operative score and a better clinical response to the treatment would support the idea that intra-articular administration of MAT could be considered in patients with very symptomatic KOA in which joint-replacement surgeries are not indicated (or accepted).
Related collections
Most cited references50
- Record: found
- Abstract: found
- Article: not found
OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis
- Record: found
- Abstract: found
- Article: not found
Adipose Mesenchymal Stromal Cell-Based Therapy for Severe Osteoarthritis of the Knee: A Phase I Dose-Escalation Trial.
- Record: found
- Abstract: found
- Article: not found