- Record: found
- Abstract: found
- Article: found
FHL2 promotes tubular epithelial‐to‐mesenchymal transition through modulating β‐catenin signalling
Read this article at
Abstract
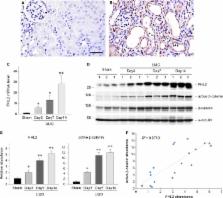
β‐Catenin signalling plays an important role in regulating tubular epithelial‐to‐mesenchymal transition ( EMT), an indispensable programme for driving renal fibrosis. As an adapter protein, four and a half LIM domain protein 2 ( FHL2) acts as a coregulator of β‐catenin in several other cell types. To determine whether FHL2 affects β‐catenin signalling and thus is involved in tubular EMT, we examined its expression and function in the process of TGF‐β1‐induced EMT. FHL2 mRNA and protein were induced by TGF‐β1 in rat tubular epithelial cells ( NRK‐52E), an effect that intracellular Smad signalling was required. Ectopic expression of FHL2 inhibited E‐cadherin and enhanced α‐smooth muscle actin (α‐ SMA) and fibronectin expression, whereas knockdown of FHL2 partially restored E‐cadherin and reduced α‐ SMA and fibronectin induction stimulated by TGF‐β1. Overexpression of FHL2 increased β‐catenin dephosphorylation (Ser37/Thr41), nuclear translocation and β‐catenin‐mediated transcription and up‐regulated expression of β‐catenin target, EMT‐related genes, such as Snail, Twist, vimentin, plasminogen activator inhibitor‐1 and matrix metalloproteinase‐7. Conversely, knockdown of FHL2 increased β‐catenin phosphorylation (Ser33/37/Thr41), decreased its nuclear translocation and inhibited β‐catenin‐mediated transcription and target genes expression. TGF‐β1 induced a FHL2/β‐catenin interaction in NRK‐52E cells, especially in the nuclei. In a mouse model of obstructive nephropathy, FHL2 mRNA and protein were induced in a time‐dependent fashion, and the extent and pattern of renal β‐catenin activation were positively correlated with FHL2 induction. Collectively, this study suggests that FHL2, via modulating β‐catenin signalling, may implicate in regulation of TGF‐β1‐mediated tubular EMT and could be a potential therapeutic target for fibrotic kidney disease.
Related collections
Most cited references25
- Record: found
- Abstract: found
- Article: not found
Origin and function of myofibroblasts in kidney fibrosis.
- Record: found
- Abstract: found
- Article: not found
Therapy for fibrotic diseases: nearing the starting line.
- Record: found
- Abstract: found
- Article: not found