- Record: found
- Abstract: found
- Article: found
Site-Specific PEGylation of Therapeutic Proteins
Read this article at
Abstract
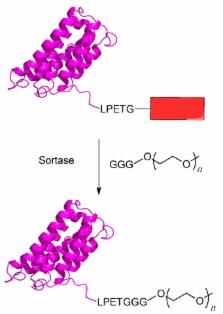
The use of proteins as therapeutics has a long history and is becoming ever more common in modern medicine. While the number of protein-based drugs is growing every year, significant problems still remain with their use. Among these problems are rapid degradation and excretion from patients, thus requiring frequent dosing, which in turn increases the chances for an immunological response as well as increasing the cost of therapy. One of the main strategies to alleviate these problems is to link a polyethylene glycol (PEG) group to the protein of interest. This process, called PEGylation, has grown dramatically in recent years resulting in several approved drugs. Installing a single PEG chain at a defined site in a protein is challenging. Recently, there is has been considerable research into various methods for the site-specific PEGylation of proteins. This review seeks to summarize that work and provide background and context for how site-specific PEGylation is performed. After introducing the topic of site-specific PEGylation, recent developments using chemical methods are described. That is followed by a more extensive discussion of bioorthogonal reactions and enzymatic labeling.
Related collections
Most cited references119
- Record: found
- Abstract: found
- Article: not found
Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B.
- Record: found
- Abstract: found
- Article: not found
Overview of tag protein fusions: from molecular and biochemical fundamentals to commercial systems.
- Record: found
- Abstract: found
- Article: not found