- Record: found
- Abstract: found
- Article: found
Grb7, a Critical Mediator of EGFR/ErbB Signaling, in Cancer Development and as a Potential Therapeutic Target
Read this article at
Abstract
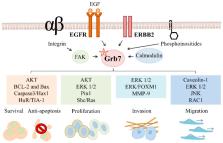
The partner of activated epidermal growth factor receptor (EGFR), growth factor receptor bound protein-7 (Grb7), a functionally multidomain adaptor protein, has been demonstrated to be a pivotal regulator for varied physiological and pathological processes by interacting with phospho-tyrosine-related signaling molecules to affect the transmission through a number of signaling pathways. In particular, critical roles of Grb7 in erythroblastic leukemia viral oncogene homolog (ERBB) family-mediated cancer development and malignancy have been intensively evaluated. The overexpression of Grb7 or the coamplification/cooverexpression of Grb7 and members of the ERBB family play essential roles in advanced human cancers and are associated with decreased survival and recurrence of cancers, emphasizing Grb7′s value as a prognostic marker and a therapeutic target. Peptide inhibitors of Grb7 are being tested in preclinical trials for their possible therapeutic effects. Here, we review the molecular, functional, and clinical aspects of Grb7 in ERBB family-mediated cancer development and malignancy with the aim to reveal alternative and effective therapeutic strategies.
Related collections
Most cited references76
- Record: found
- Abstract: found
- Article: found
Emerging Roles of Focal Adhesion Kinase in Cancer
- Record: found
- Abstract: found
- Article: not found
Intrinsic Subtype Switching and Acquired ERBB2/HER2 Amplifications and Mutations in Breast Cancer Brain Metastases.
- Record: found
- Abstract: found
- Article: not found