Introduction
Heart failure (HF) continues to pose a high burden for patients and the healthcare system alike. HF affects 1–2% of the US population, approximately 6 million people, and has an 5-year mortality approaching approximately 50%; therefore, it is an important focus for medicine [1]. Health care costs associated with HF are high, with an estimated cost per patient year of $24,000, most of which comprises HF hospital admissions [2]. Given the high overall morbidity, mortality, and cost of HF, effective and affordable therapies are in high demand.
In the past few years, a series of randomized controlled trials examining sodium-glucose cotransporter-2 inhibitors (SGLT2i) in patients with HF and reduced ejection fraction showed promising results. Several of these large studies have indicated that treatment with SGLT2i, compared with placebo, decreased cardiovascular death and readmissions, and improves quality of life scores [3–7]. Although SGLT2i are the medical therapy most recently included in guideline directed therapy for HF, mineralocorticoid receptor antagonists (MRAs) have been an established HF treatment for decades. MRAs have been shown to decrease mortality and readmissions in patients with HF [8–10].
Because the cost of therapies to both individual patients and the healthcare system is an important determinant of successful treatment, evaluating the cost-effectiveness of this new class of medications is important. This study was aimed at assessing the cost-effectiveness of the SGLT2i compared with the usual standard of care using MRAs.
Materials and Methods
Model Overview
We developed a Markov model with yearly cycles to assess the cost-effectiveness of MRAs versus SGLT2i in patients with heart failure with reduced ejection fraction (HFrEF). The model had two states (HFrEF and dead), which simulated the progression of HFrEF (Figure 1). All patients entered the model in the HFrEF state and could progress to the death state in any simulated year. Every year, patients had a likelihood of encountering hospitalization events, which were associated with additional costs and decreased health utility. The simulation was conducted for a 5 year window, with costs and quality-adjusted life-years (QALYs) discounted at 5% yearly. The primary outcome was the incremental net benefit (INB), which was calculated as λ×Δ effectiveness - Δ cost. Δ Effectiveness denotes the difference in QALYs gained between MRAs and SGLT2i, and Δ cost denotes the difference in medical costs between MRAs and SGLT2i. We used a willingness to pay (λ) of $50,000 per QALY in this study [1]. This economic simulation analysis was exempt from institutional review board approval and informed consent. The key data inputs for the model are summarized in Table 1, and details of the data sources for model inputs are provided below.

Markov Model Diagram.
Patients occupy health states, as shown in ovals. Patients transition among health states, as represented by arrows based on transition probabilities.
Key Data Inputs for the Model.
| Annual event probabilities, % | Value (range) | Source |
|---|---|---|
| SGLT2i | ||
| All-cause hospitalization | 0.549 | Packer 2020 [7] (EMPEROR-Reduced) |
| All-cause mortality | 0.100 | Packer 2020 [7] (EMPEROR-Reduced) |
| MRAs vs SGLT2i, RR (95% CI) | ||
| All-cause hospitalization* | 1.05 (0.84–1.31) | Network meta-analysis of trials |
| All-cause mortality | 0.91 (0.78–1.06) | Network meta-analysis of trials |
| Annual cost, median (IQR), $ | ||
| Background cost | 3557 (1934–11,574) | Urbich et al. 2020 [2] |
| All-cause hospitalization | 20,826 (18,779–29,045) | Urbich et al.2020 [2] |
| SGLT2i | 6297.47 | CMS 2020 [11] |
| MRAs | 821.25 | CMS 2020 [11] |
| Utilities | ||
| Baseline utility | 0.74 | Eurich et al. 2006 [12] |
| Hospitalization, % baseline utility | −29 | Ambrosy et al. 2016 [13] |
| Discount rate, median (IQR), % | 3 (0 - 5) | Attema et al. 2018 [14] |
MRAs, mineralocorticoid antagonists; SGLT2i, sodium-glucose cotransporter 2 inhibitors; RR, risk ratio; CI, confidence interval; IQR, Interquartile range.
*We assumed similar risks for all-cause hospitalization and HF hospitalization between MRAs and SGLT2 inhibitors.
Simulation Sample
The cohort of patients modeled in the analysis came from the Empagliflozin Outcome Trial in Patients with Chronic Heart Failure and Reduced Ejection Fraction (EMPEROR-Reduced) trial [4]. This trial included patients (≥18 years of age) who had chronic HF (functional class II, III, or IV) with a left ventricular ejection fraction of 40% or less and an N-terminal pro–B-type natriuretic peptide level (>300 pg/mL or >900 pg/mL for patients with atrial fibrillation at baseline). Patients with disorders that could potentially alter their clinical course independently of HF, or with had any condition that might jeopardize patients’ safety or limit their participation in the trial, were excluded.
Data Analysis
All-Cause Mortality and Hospitalization Associated with SGLT2i and MRAs
Because a head-to-head trial between MRAs and SGLT2i was lacking, we performed a frequentist network meta-analysis of the Randomized Aldactone Evaluation Study (RALES) [8], the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) [9], the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure study (EMPHASIS-HF) [10], the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure trial (DAPA-HF) [5], and the EMPEROR-Reduced trial [4] to estimate the risk ratio (RR) of all-cause mortality and HF hospitalization (HHF) between MRAs and SGLT2i. The characteristics of five trials are presented in Supplementary Table S1. Because the RR for all-cause hospitalization between MRAs and SGLT2i was unavailable, we assumed that the risks for all-cause hospitalization and HHF between MRAs and SGLT2i were similar in this study.
The annual probabilities of all-cause mortality and all-cause hospitalization for patients receiving SGLT2i were from the EMPEROR-Reduced trial. The risks of all-cause mortality and all-cause hospitalization in the empagliflozin group were 13.4% (249/1863) and 73.2% (1364/1863), respectively, during a median of 16 months’ follow-up [4]. Accordingly, the estimated 1-year probabilities of all-cause mortality and all-cause hospitalization in the SGLT2i group were 10.0% and 54.9%, respectively. For patients treated with MRAs, the probability of all-cause mortality and all-cause hospitalization were calculated on the basis of the risk of SGLT2i multiplied by the RR calculated from the network meta-analysis described above. In this model, we hypothesized that the risks of all-cause mortality and all-cause hospitalization were constant during the 5 years.
Costs and Health Utilities
The cost inputs included the cost of all-cause hospitalization, background treatments, and target drugs. The cost of hospitalization and background treatments was extracted from a systematic review [2]. The median cost for all-cause hospitalization was $20,826 (interquartile range (IQR), $18,779–$29,045). We estimated the annual background treatment cost to be $3557 (IQR, $1934–$11,574) [2]. The annual costs of SGLT2i and MRAs were calculated on the basis of data from Medicare Part D Spending reported by CMS in 2020 [11]. The 2020 annual costs of SGLT2i and MRAs were $6297.47 and $821.25, respectively. The baseline utility for patients with HFrEF was estimated according to a previous report [15]. A utility decrement of 29% was applied to patients with all-cause hospitalization [16].
Sensitivity Analyses
We performed two clinically relevant scenario analyses. First, we assumed an optimistic scenario for SGLT2i, in which we used the higher bound of CI for all-cause hospitalization and all-cause mortality of RR from network meta-analysis. Second, we assumed an optimistic scenario for MRA, in which we used the lower bound of CI for all-cause hospitalization and all-cause mortality of RR from network meta-analysis. We also performed one-way sensitivity analyses to test the robustness of the results and evaluate the effects of uncertainty by changing key data inputs one at a time in the model. The parameters were varied across the 95% confidence interval (CI) or IQR where available (Table 1). Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) was used to create and analyze the model.
Results
This study reviewed data from the RALES [8], EPHESUS [9], EMPHASIS [10], DAPA-HF [5], and EMPORER-Reduced [4] trials. RR values were calculated for cardiovascular death and HF exacerbations (CV death-HHF), all-cause mortality, and HHF.
The results from this network meta-analysis are presented in Supplementary Table S2. The relative risk of CV death-HHF reduction for MRAs (RR 0.75; 95% confidence interval (CI) 0.61–0.92) and SGLT2i (RR 0.72; 95% CI 0.6–0.98) indicated no significant difference between treatments (RR 1.04; 95% CI 0.82–1.31). MRAs were associated with a lower but non-significant risk of all-cause mortality (RR 0.91; 95% CI 0.78–1.06) and a slightly higher but non-significant risk of HHF (RR 1.05; 95% CI 0.84–1.31) than SGLT2i.
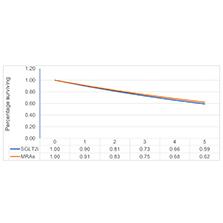
Costs for the therapies were broken-down into annual background cost ($3557), hospitalization cost ($20,826), and annual cost of SGLT2i ($5682) and MRAs ($399). Over the 5-year simulation period (Figure 2), the 5-year survival rates for MRAs and SGLT2i were 62% and 59%, respectively. The results are presented in Table 2. The patients in the MRA group were projected to gain 2.42 QALYs, whereas those in the SGLT2i group were projected to gain 2.37 QALYs, thereby resulting in a QALY difference of 0.05. The total medical cost for MRAs and SGLT2i users was $64,802 and $82,754, respectively, thus indicating a difference of $17,952. When a threshold of $50,000 per QALY gained was applied, the INB was $20,565. An INB greater than 0 indicated that MRAs were a cost-effective option with respect to SGLT2i.

Survival Curves by Treatment Strategy.
Estimates of the probability of being alive at any given time over 5 years are shown.
Total Cost, Health Effects, and Incremental Net Benefit.
| Cost, $ | QALYs | ||||
|---|---|---|---|---|---|
| Total | Δ Cost | Total | Δ Effectiveness | INB, $* | |
| SGLT2i | 82,754 | 2.37 | |||
| MRAs | 64,802 | −17,952 | 2.42 | 0.05 | 20,564 |
MRAs, mineralocorticoid antagonists; SGLT2i, sodium-glucose cotransporter 2 inhibitors; QALYs, quality-adjusted life-years; INB, incremental net benefit.
*INB = λ × Δ effectiveness − Δ cost. Δ Effectiveness denotes the difference in QALYs gained between MRAs and SGLT2i, Δ Cost denotes the difference in medical cost between MRAs and SGLT2i, and λ denotes the willingness-to-pay of $50,000 per QALYs in this study.
Sensitivity Analyses
The results of the one-way sensitivity analysis of MRAs versus SGLT2i are shown in Table 3. The INBs from the one-way sensitivity analyses remained above $0, thus suggesting that our conclusion was robust to parameter uncertainties. When the mortality was tested over the 95% CI from network meta-analysis, the INB ranged from $16,606 to $24,152. The range of INB was from $4046 to $33,907 according to the 95% CI of RR for risk of hospitalization. The results were not sensitive to the cost of hospitalization (range: $19,365–$20,863) or the discount rate (range: $20,006–$21,484).
Sensitivity Analyses.
| Parameters | INB, $ | |||
|---|---|---|---|---|
|
| ||||
| One-way sensitivity analyses | ||||
|
| ||||
| Lower limit | Upper limit | Lower limit | Upper limit | |
| RR of all-cause hospitalization (MRAs vs SGLT2i) | 0.84 | 1.31 | 4046 | 33,907 |
| RR of all-cause mortality (MRAs vs SGLT2i) | 0.78 | 1.06 | 16,606 | 24,152 |
| Background cost, $ | 1934 | 11,574 | 20,020 | 20,675 |
| Cost of hospitalization, $ | 18,779 | 29,045 | 19,365 | 20,863 |
| Discount rate, % | 0 | 5 | 20,006 | 21,484 |
| Scenario analyses | ||||
| Optimistic SGLT2i scenario* | - | - | 562 | |
| Optimistic MRA scenario† | - | - | 37,836 | |
RR, risk ratio; INB, incremental net benefit.
*The optimistic scenario for SGLT2i, in which we used the higher bound of CI for all-cause hospitalization (RR = 1.31) and all-cause mortality (RR = 1.06) of the RR from network meta-analysis.
†The optimistic scenario for MRAs, in which we used the lower bound of CI for all-cause hospitalization (RR = 0.84) and all-cause mortality (RR = 0.78) of the RR from network meta-analysis.
The results appeared to be robust in the scenario analysis (Table 3). In the optimistic SGLT2i scenario, the higher bound of the 95% CI of RRs for hospitalization and all-cause mortality between MRAs and SGLT2i were 1.31 and 1.06, respectively. The INB of MRAs compared with SGLT2i was $562. In the optimistic MRAs scenario, the lower bound of the 95% CI of RRs for both hospitalization and all-cause mortality between MRAs and SGLT2i was 0.84 and 0.78, respectively. The INB of MRAs compared with SGLT2i was $37,836.
Discussion
The median annual cost of SGLT2i in 2019 exceeded $5000 [11]. Although this class of medications is clearly exciting because it confers benefits for diabetes, renal disease, and heart disease, providing care in the current medical system requires consideration of the cost of care.
Physicians have several validated and guideline recommended medical therapy (GDMT) options for treating HFrEF, including beta blockers, angiotensin converting enzyme blockers, MRAs, neprilysin inhibitors, and most recently SGLT2i [17]. Ideally, all patients with HFrEF should be able to tolerate, afford, and maintain adherence to all GDMT. However, full compliance with GDMT is rare for multiple potential reasons, including medical tolerance, hemodynamic limitations, polypharmacy decreasing compliance, and medication cost. Prescription complexity and polypharmacy are common in patients with HF, given the recommended therapies as well as therapies for common comorbidities [18]. This complexity can decrease medication adherence [13]. Medication cost can also lead to medication nonadherence. Approximately 20%–35% of patients report decreasing medication adherence because of medication cost, and this pattern is observed across healthcare systems and insurance coverage levels [19].
Prescribers and patients must choose a medication regimen that will be efficacious in treating medical disease, have an appropriate adverse effect profile, and importantly have reasonably low complexity and cost to support patient adherence. Cost benefit analysis therefore could help inform treatment decision-making for conditions such as HF, in which clinicians and patients often must choose between two or more effective therapies.
The DAPA-HF [5] and EMPORER-Reduced [4] trials showed benefits of SGLT2i, and the RALES [8], EPHESUS [9], and EMPHASIS [10] trials showed benefits of MRAs in patients with HFrEF. Since these trials, several studies have examined the costs associated with these therapies. A follow up study examining the cost-effectiveness of SGLT2i in Europe has modeled an improvement in QALYs with dapagliflozin compared with standard therapy from 4.13 to 4.61, and a cost-effectiveness ratio of £5822/QALY in the United Kingdom, €5379/QALY in Germany, and €9406/QALY in Spain. The authors have argued that, given a willingness to pay threshold of €20,000 or £20,000 per QALY, treatment with dapagliflozin for HF was cost-effective with respect to the standard of care [20]. A total of 71.5% of the treatment group and 70.6% of the control group in that study were treated with MRA, thus suggesting the acceptable cost benefit of adding SGLT2i to a population already relatively compliant with recommended GDMT.
A research group from Australia has examined the cost-effectiveness of empagliflozin and eplerenone compared with the standard of care. Their model has indicated a cost-effectiveness of AU$37,452 per QALY gained with eplerenone and AU$12,482 per QALY gained with dapagliflozin, both of which were considered acceptable, on the basis of a willingness to pay ratio of AU$50,0000/QALY in Australia [21]. These studies suggest that both MRAs and SGLT2i, as compared with the standard of care, are cost-effective for patients with HFrEF. However, choosing between these treatments according to a cost-effectiveness perspective had not been examined.
Both SGLT2i and MRAs have been shown to be beneficial in HFrEF, are guideline recommended first line therapies, and have been argued to be cost-effective, according to willingness to pay ratios. However, as suggested by several studies, the use of GDMT, including beta blockers, angiotensin converting enzyme blockers, and MRAs, is low [22, 23]. This low use rate may be due to many factors and challenges in our health system, including medication regimen complexity, polypharmacy, and prohibitive cost of medications.
The above analysis showed that the QALYs gained, modeled over 5 years, remained relatively the same between SGLT2i and MRA. These findings would suggest that both medications have similar efficacy. However, SGLT2i have a substantially higher cost. The cost difference over the modeled 5 years was $17,952, in favor of MRA. This higher health care associated cost with SGLT2i, of approximately $3500 per year in the Markov model, was not associated with any excess benefit. In fact, the QALYs gained in our model trended in favor of MRA.
Similar benefits in terms of QALYs were observed for MRAs and SGLT2i. However, because MRAs have substantially lower annual cost than SGLT2i, our findings suggested that MRAs were the more cost-effective option. In a reality in which many patients are unable to afford all recommended medications, if a provider were to have to choose between prescribing an MRA or an SGLT2i for a patient with HFrEF, with all other medical considerations being equal, we recommend MRAs as the more cost-effective choice.
This study has several limitations. First, the progression of HFrEF was simulated with a two-state model (i.e., alive with or without a hospitalization, and dead), without taking into account other adverse events (e.g., ketoacidosis). Second, although the inputs were derived from reliable sources such as the EMPORER-Reduced trial, limited additional data were available on the effectiveness of MRAs versus SGLT2i, and no real-world evidence has currently been established. In addition, given the unavailability of risk data on of all-cause hospitalization between MRAs and SGLT2i, we assumed that the risk of all-cause hospitalization between MRAs and SGLT2i was similar to that of HHF. However, the one-way sensitivity indicated the robustness of our findings. Third, owing to the absence of long-term follow-up data, we assumed that the trends observed in the EMPORER-Reduced trial, such as decreases in all-cause mortality and hospitalizations, would continue beyond the end of the trial. Fourth, in our model, the transition probability was fixed and was influenced by age. Finally, we were unable to account for patient adherence and consequent effects on outcomes, because such data are not currently available. Therefore, future studies on real-world effectiveness and cost-effectiveness should consider regimen adherence.
Conclusion
MRAs and SGLT2 both showed benefits in patients with HFrEF, and are recommended as part of standard GDMT. However, owing to the current restrictions of cost, pill burden, and care access in the current medical system, not every patient receives all recommended therapies. The cost of care and medications was an important factor that unfortunately affects medical decisions and prescribing patterns; therefore, the cost-effectiveness of HF medications was a useful decision tool. MRAs and SGLT2i provided similar benefits. However, MRAs were a more cost-effective treatment option than SGLT2i for patients with HFrEF.