- Record: found
- Abstract: found
- Article: found
The Serum S100B Level as a Biomarker of Enteroglial Activation in Patients with Ulcerative Colitis
Read this article at
Abstract
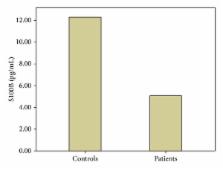
Objective. Recent studies have demonstrated that enteric glial cells (EGC) participate in the homeostasis of the gastrointestinal tract. This study investigated whether enteroglial markers, including S100B protein and glial fibrillary acidic protein (GFAP), can serve as noninvasive indicators of EGC activation and disease activity in UC patients. Methods. This clinical prospective study included 35 patients with UC and 40 age- and sex-matched controls. The diagnosis of UC was based on standard clinical, radiological, endoscopic, and histological criteria. Clinical disease activity was evaluated using the Modified Truelove-Witts Severity Index. Serum samples were analyzed for human GFAP and S100B using commercial enzyme-linked immunosorbent assay kits. Results. GFAP was not detected in the serum of either UC patients or controls ( P > 0.05). However, we found a significant ( P < 0.001) decrease in the serum S100B levels in the UC patients. No correlation between the serum S100B level and the disease activity or duration was observed ( P > 0.05). The serum S100B levels did not differ between UC patients with active disease (24 patients, 68.6%) or in remission (11 patients, 31.4%) ( P > 0.05). Conclusions. Ulcerative colitis patients had significantly lower serum S100B levels, while GFAP was of no diagnostic value in UC patients.
Related collections
Most cited references34
- Record: found
- Abstract: not found
- Article: not found
A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis.
- Record: found
- Abstract: found
- Article: not found
Cyclosporine in severe ulcerative colitis refractory to steroid therapy.
- Record: found
- Abstract: found
- Article: not found