- Record: found
- Abstract: found
- Article: found
NatF Contributes to an Evolutionary Shift in Protein N-Terminal Acetylation and Is Important for Normal Chromosome Segregation
Read this article at
Abstract
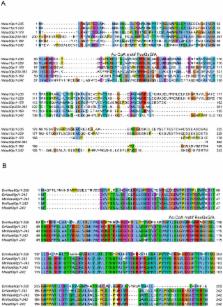
N-terminal acetylation (N-Ac) is a highly abundant eukaryotic protein modification. Proteomics revealed a significant increase in the occurrence of N-Ac from lower to higher eukaryotes, but evidence explaining the underlying molecular mechanism(s) is currently lacking. We first analysed protein N-termini and their acetylation degrees, suggesting that evolution of substrates is not a major cause for the evolutionary shift in N-Ac. Further, we investigated the presence of putative N-terminal acetyltransferases (NATs) in higher eukaryotes. The purified recombinant human and Drosophila homologues of a novel NAT candidate was subjected to in vitro peptide library acetylation assays. This provided evidence for its NAT activity targeting Met-Lys- and other Met-starting protein N-termini, and the enzyme was termed Naa60p and its activity NatF. Its in vivo activity was investigated by ectopically expressing human Naa60p in yeast followed by N-terminal COFRADIC analyses. hNaa60p acetylated distinct Met-starting yeast protein N-termini and increased general acetylation levels, thereby altering yeast in vivo acetylation patterns towards those of higher eukaryotes. Further, its activity in human cells was verified by overexpression and knockdown of hNAA60 followed by N-terminal COFRADIC. NatF's cellular impact was demonstrated in Drosophila cells where NAA60 knockdown induced chromosomal segregation defects. In summary, our study revealed a novel major protein modifier contributing to the evolution of N-Ac, redundancy among NATs, and an essential regulator of normal chromosome segregation. With the characterization of NatF, the co-translational N-Ac machinery appears complete since all the major substrate groups in eukaryotes are accounted for.
Author Summary
Small chemical groups are commonly attached to proteins in order to control their activity, localization, and stability. An abundant protein modification is N-terminal acetylation, in which an N-terminal acetyltransferase (NAT) catalyzes the transfer of an acetyl group to the very N-terminal amino acid of the protein. When going from lower to higher eukaryotes there is a significant increase in the occurrence of N-terminal acetylation. We demonstrate here that this is partly because higher eukaryotes uniquely express NatF, an enzyme capable of acetylating a large group of protein N-termini including those previously found to display an increased N-acetylation potential in higher eukaryotes. Thus, the current study has possibly identified the last major component of the eukaryotic machinery responsible for co-translational N-acetylation of proteins. All eukaryotic proteins start with methionine, which is co-translationally cleaved when the second amino acid is small. Thereafter, NatA may acetylate these newly exposed N-termini. Interestingly, NatF also has the potential to act on these types of N-termini where the methionine was not cleaved. At the cellular level, we further found that NatF is essential for normal chromosome segregation during cell division.
Related collections
Most cited references50
- Record: found
- Abstract: found
- Article: not found
Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics.
- Record: found
- Abstract: not found
- Article: not found
Improved visualization of protein consensus sequences by iceLogo.
- Record: found
- Abstract: found
- Article: not found