- Record: found
- Abstract: not found
- Article: not found
Emerging and Re-emerging Warheads for Targeted Covalent Inhibitors: An Update
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Related collections
Most cited references531
- Record: found
- Abstract: found
- Article: not found
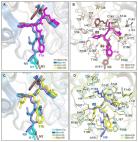
The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity
Jude Canon, Karen Rex, Anne Saiki … (2019)
- Record: found
- Abstract: found
- Article: found
Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease
Wenhao Dai, Bing Zhang, Haixia Su … (2020)
- Record: found
- Abstract: found
- Article: not found