- Record: found
- Abstract: found
- Article: found
A Type III Effector NleF from EHEC Inhibits Epithelial Inflammatory Cell Death by Targeting Caspase-4
Read this article at
Abstract
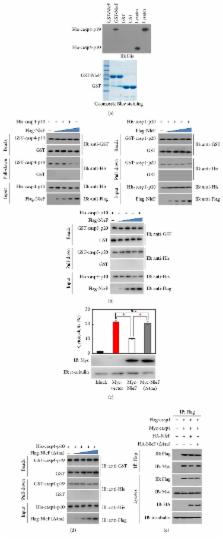
Enterohemorrhagic E. coli (EHEC) is a highly pathogenic bacterial strain capable of inducing severe gastrointestinal disease. Here, we show that EHEC uses the T3SS effector NleF to counteract the host inflammatory response by dampening caspase-4-mediated inflammatory epithelial cell death and by preventing the production of IL-1 β. The other two inflammatory caspases, caspase-1 and caspase-5, are not involved in EHEC Δ nleF-induced inflammatory cell death. We found that NleF not only interrupted the heterodimerization of caspase-4-p19 and caspase-4-p10, but also inhibited the interaction of caspase-1 and caspase-4. The last four amino acids of the NleF carboxy terminus are essential in inhibiting caspase-4-dependent inflammatory cell death.
Related collections
Most cited references24
- Record: found
- Abstract: found
- Article: not found
Innate and adaptive immunity in inflammatory bowel disease.
- Record: found
- Abstract: found
- Article: not found
Noncanonical inflammasome activation of caspase-4/caspase-11 mediates epithelial defenses against enteric bacterial pathogens.
- Record: found
- Abstract: found
- Article: not found