- Record: found
- Abstract: found
- Article: not found
Signalling through C-type lectin receptors: shaping immune responses
Read this article at
Key Points
-
Crosstalk between pattern recognition receptors (PRRs) expressed by dendritic cells orchestrates T helper (T H) cell differentiation through the induction of specific cytokine expression profiles, tailored to invading pathogens. C-type lectin receptors (CLRs) have an important role in orchestrating the induction of signalling pathways that regulate adaptive immune responses.
-
CLRs can control adaptive immunity at various levels by inducing signalling on their own, through crosstalk with other PRRs or by inducing carbohydrate-specific signalling pathways.
-
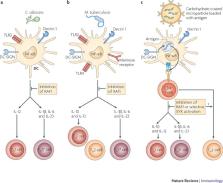
DC-specific ICAM3-grabbing non-integrin (DC-SIGN) interacts with mannose-carrying pathogens including Mycobacterium tuberculosis, HIV-1, measles virus and Candida albicans to activate the serine/threonine protein kinase RAF1. RAF1 signalling leads to the acetylation of Toll-like receptor (TLR)-activated nuclear factor-κB (NF-κB) subunit p65 and affects cytokine expression, such as inducing the upregulation of interleukin-10 (IL-10).
-
DC-associated C-type lectin 1 (dectin 1) triggering by a broad range of fungal pathogens, such as C. albicans, Aspergillus fumigatus and Pneumocystis carinii, results in protective antifungal immunity through the crosstalk of two independent signalling pathways — one through spleen tyrosine kinase (SYK) and one through RAF1 — that are essential for the expression of T H1 and T H17 cell polarizing cytokines.
-
Crosstalk between the SYK and RAF1 pathways is both synergistic and antagonizing to fine-tune NF-κB activity: although Ser276 phosphorylation of p65 leads to enhanced transcriptional activity of p65 itself through acetylation, it also inhibits the transcriptional activity of the NF-κB subunit RELB by sequestering it in p65–RELB dimers, which are transcriptionally inactive.
-
The diversity in CLR-mediated signalling provides some major challenges for the researches to elucidate and manipulate the signalling properties of this exciting family of receptors. However, the recent advances strongly support the use of CLR targeting vaccination strategies using dendritic cells to induce or redirect adaptive immune responses as well as improve antigen delivery.
Abstract
Here, Teunis Geijtenbeek and Sonja Gringhuis discuss the role of the signalling pathways induced by C-type lectin receptors in determining T helper cell lineage commitment and describe how these pathways can be exploited for the development of new vaccination strategies.
Abstract
C-type lectin receptors (CLRs) expressed by dendritic cells are crucial for tailoring immune responses to pathogens. Following pathogen binding, CLRs trigger distinct signalling pathways that induce the expression of specific cytokines which determine T cell polarization fates. Some CLRs can induce signalling pathways that directly activate nuclear factor-κB, whereas other CLRs affect signalling by Toll-like receptors. Dissecting these signalling pathways and their effects on host immune cells is essential to understand the molecular mechanisms involved in the induction of adaptive immune responses. In this Review we describe the role of CLR signalling in regulating adaptive immunity and immunopathogenesis and discuss how this knowledge can be harnessed for the development of innovative vaccination approaches.
Related collections
Most cited references90
- Record: found
- Abstract: found
- Article: not found
Taking dendritic cells into medicine.
- Record: found
- Abstract: found
- Article: not found
IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge.
- Record: found
- Abstract: found
- Article: not found
