- Record: found
- Abstract: found
- Article: found
SIRT1 Activators Suppress Inflammatory Responses through Promotion of p65 Deacetylation and Inhibition of NF-κB Activity
Read this article at
Abstract
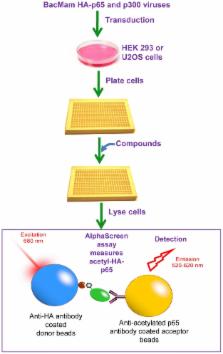
Chronic inflammation is a major contributing factor in the pathogenesis of many age-associated diseases. One central protein that regulates inflammation is NF-κB, the activity of which is modulated by post-translational modifications as well as by association with co-activator and co-repressor proteins. SIRT1, an NAD +-dependent protein deacetylase, has been shown to suppress NF-κB signaling through deacetylation of the p65 subunit of NF-κB resulting in the reduction of the inflammatory responses mediated by this transcription factor. The role of SIRT1 in the regulation of NF-κB provides the necessary validation for the development of pharmacological strategies for activating SIRT1 as an approach for the development of a new class of anti-inflammatory therapeutics. We report herein the development of a quantitative assay to assess compound effects on acetylated p65 protein in the cell. We demonstrate that small molecule activators of SIRT1 (STACs) enhance deacetylation of cellular p65 protein, which results in the suppression of TNFα-induced NF-κB transcriptional activation and reduction of LPS-stimulated TNFα secretion in a SIRT1-dependent manner. In an acute mouse model of LPS-induced inflammation, the STAC SRTCX1003 decreased the production of the proinflammatory cytokines TNFα and IL-12. Our studies indicate that increasing SIRT1-mediated NF-κB deacetylation using small molecule activating compounds is a novel approach to the development of a new class of therapeutic anti-inflammatory agents.
Related collections
Most cited references48
- Record: found
- Abstract: found
- Article: not found
Mammalian sirtuins: biological insights and disease relevance.
- Record: found
- Abstract: found
- Article: not found
Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes.
- Record: found
- Abstract: found
- Article: not found