- Record: found
- Abstract: found
- Article: not found
Disease-drug and drug-drug interaction in COVID-19: Risk and assessment
Read this article at
Abstract
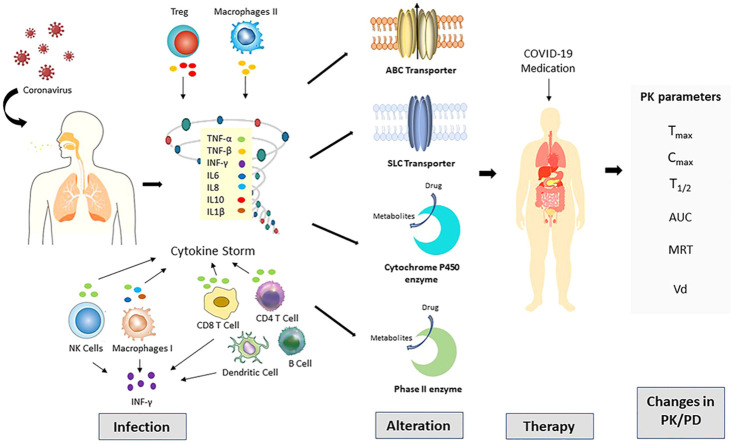
COVID-19 is announced as a global pandemic in 2020. Its mortality and morbidity rate are rapidly increasing, with limited medications. The emergent outbreak of COVID-19 prompted by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) keeps spreading. In this infection, a patient's immune response plays pivotal role in the pathogenesis. This inflammatory factor was shown by its mediators that, in severe cases, reach the cytokine at peaks. Hyperinflammatory state may sparks significant imbalances in transporters and drug metabolic machinery, and subsequent alteration of drug pharmacokinetics may result in unexpected therapeutic response. The present scenario has accounted for the requirement for therapeutic opportunities to relive and overcome this pandemic. Despite the diminishing developments of COVID-19, there is no drug still approved to have significant effects with no side effect on the treatment for COVID-19 patients. Based on the evidence, many antiviral and anti-inflammatory drugs have been authorized by the Food and Drug Administration (FDA) to treat the COVID-19 patients even though not knowing the possible drug-drug interactions (DDI). Remdesivir, favipiravir, and molnupiravir are deemed the most hopeful antiviral agents by improving infected patient’s health. Dexamethasone is the first known steroid medicine that saved the lives of seriously ill patients. Some oligopeptides and proteins have also been using. The current review summarizes medication updates to treat COVID-19 patients in an inflammatory state and their interaction with drug transporters and drug-metabolizing enzymes. It gives an opinion on the potential DDI that may permit the individualization of these drugs, thereby enhancing the safety and efficacy.
Graphical Abstract
Related collections
Most cited references318
- Record: found
- Abstract: found
- Article: not found
Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China
- Record: found
- Abstract: found
- Article: not found
Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study
- Record: found
- Abstract: found
- Article: not found