- Record: found
- Abstract: found
- Article: found
Canagliflozin mediates tumor suppression alone and in combination with radiotherapy in non‐small cell lung cancer (NSCLC) through inhibition of HIF‐1α

Read this article at
Abstract
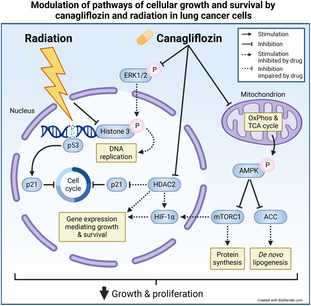
Non‐small cell lung cancer (NSCLC) has a poor prognosis, and effective therapeutic strategies are lacking. The diabetes drug canagliflozin inhibits NSCLC cell proliferation and the mammalian target of rapamycin (mTOR) pathway, which mediates cell growth and survival, but it is unclear whether this drug can enhance response rates when combined with cytotoxic therapy. Here, we evaluated the effects of canagliflozin on human NSCLC response to cytotoxic therapy in tissue cultures and xenografts. Ribonucleic acid sequencing (RNA‐seq), real‐time quantitative PCR (RT‐qPCR), metabolic function, small interfering ribonucleic acid (siRNA) knockdown, and protein expression assays were used in mechanistic analyses. We found that canagliflozin inhibited proliferation and clonogenic survival of NSCLC cells and augmented the efficacy of radiotherapy to mediate these effects and inhibit NSCLC xenograft growth. Canagliflozin treatment alone moderately inhibited mitochondrial oxidative phosphorylation and exhibited greater antiproliferative capacity than specific mitochondrial complex‐I inhibitors. The treatment downregulated genes mediating hypoxia‐inducible factor (HIF)‐1α stability, metabolism and survival, activated adenosine monophosphate‐activated protein kinase (AMPK) and inhibited mTOR, a critical activator of hypoxia‐inducible factor‐1α (HIF‐1α) signaling. HIF‐1α knockdown and stabilization experiments suggested that canagliflozin mediates antiproliferative effects, in part, through suppression of HIF‐1α. Transcriptional regulatory network analysis pinpointed histone deacetylase 2 ( HDAC2), a gene suppressed by canagliflozin, as a key mediator of canagliflozin's transcriptional reprogramming. HDAC2 knockdown eliminated HIF‐1α levels and enhanced the antiproliferative effects of canagliflozin. HDAC2‐regulated genes suppressed by canagliflozin are associated with poor prognosis in several clinical NSCLC datasets. In addition, we include evidence that canagliflozin also improves NSCLC response to chemotherapy. In summary, canagliflozin may be a promising therapy to develop in combination with cytotoxic therapy in NSCLC.
Abstract
The diabetes drug canagliflozin suppresses non‐small cell lung cancer growth and enhances the efficacy of radiotherapy. Canagliflozin inhibits the cell cycle, mitochondrial respiration, and growth‐promoting pathways. Here, the authors uncovered that canagliflozin mediates its effects partly by suppressing hypoxia‐inducible factor‐1α and histone deacetylase 2.
Related collections
Most cited references63
- Record: found
- Abstract: found
- Article: not found
Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries

- Record: found
- Abstract: found
- Article: found
Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2
- Record: found
- Abstract: found
- Article: not found