- Record: found
- Abstract: found
- Article: found
Pien-Tze-Huang attenuates neuroinflammation in cerebral ischaemia-reperfusion injury in rats through the TLR4/NF-κB/MAPK pathway
Read this article at
Abstract
Context
Pien-Tze-Huang (PTH) is traditionally applied to treat various inflammation-related diseases including stroke. However, literature regarding the anti-inflammatory effects and possible mechanisms of PTH in ischaemic stroke is unavailable.
Objective
This study investigates the anti-inflammatory effects and its underlying mechanism of PTH on ischaemic stroke.
Materials and methods
Cerebral ischaemia-reperfusion injury was induced through 2 h middle cerebral artery occlusion (MCAO) followed by 24 h reperfusion in male Sprague-Dawley (SD) rats receiving oral pre-treatment with PTH (180 mg/kg) for 4 days. TLR4 antagonist TAK-242 (3 mg/kg) was injected intraperitoneally at 1.5 h after MCAO. MRI, HE staining, qRT-PCR, western blot, and immunofluorescence methods were employed.
Results
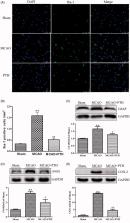
PTH treatment markedly reduced cerebral infarct volume (by 51%), improved neurological function (by 33%), and ameliorated brain histopathological damage in MCAO rats. It also reduced the levels of four inflammatory mediators including IL-1β (by 70%), IL-6 (by 78%), TNF-α (by 60%) and MCP-1 (by 58%); inhibited microglia and astrocyte activation; and decreased protein expression of iNOS and COX-2 in injured brains. Moreover, PTH down-regulated the protein expressions of TLR4, MyD88, and TRAF6; reduced the expression and nuclear translocation of NF-κB; and lowered the protein expressions of p-ERK1/2, p-JNK, and p-p38. Similar effects were observed in MCAO rats with TAK-242 treatment. However, combined administration of PTH and TAK-242 did not significantly reinforce the anti-inflammatory effects of PTH.
Related collections
Most cited references32
- Record: found
- Abstract: not found
- Article: not found
Heart Disease and Stroke Statistics—2018 Update: A Report From the American Heart Association
- Record: found
- Abstract: found
- Article: not found
Ischemia and reperfusion--from mechanism to translation.
- Record: found
- Abstract: found
- Article: not found