- Record: found
- Abstract: found
- Article: found
Self-Assembly, Bioactivity, and Nanomaterials Applications of Peptide Conjugates with Bulky Aromatic Terminal Groups

Read this article at
Abstract
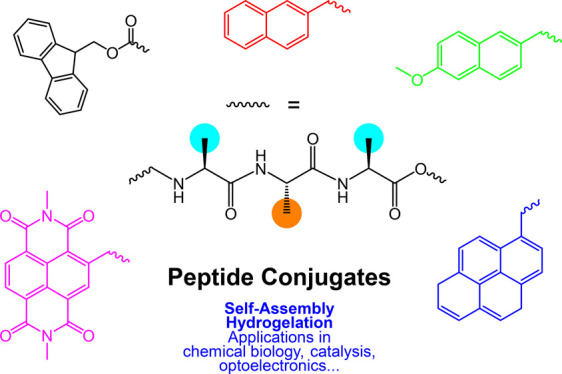
The self-assembly and structural and functional properties of peptide conjugates containing bulky terminal aromatic substituents are reviewed with a particular focus on bioactivity. Terminal moieties include Fmoc [fluorenylmethyloxycarbonyl], naphthalene, pyrene, naproxen, diimides of naphthalene or pyrene, and others. These provide a driving force for self-assembly due to π-stacking and hydrophobic interactions, in addition to the hydrogen bonding, electrostatic, and other forces between short peptides. The balance of these interactions leads to a propensity to self-assembly, even for conjugates to single amino acids. The hybrid molecules often form hydrogels built from a network of β-sheet fibrils. The properties of these as biomaterials to support cell culture, or in the development of molecules that can assemble in cells (in response to cellular enzymes, or otherwise) with a range of fascinating bioactivities such as anticancer or antimicrobial activity, are highlighted. In addition, applications of hydrogels as slow-release drug delivery systems and in catalysis and other applications are discussed. The aromatic nature of the substituents also provides a diversity of interesting optoelectronic properties that have been demonstrated in the literature, and an overview of this is also provided. Also discussed are coassembly and enzyme-instructed self-assembly which enable precise tuning and (stimulus-responsive) functionalization of peptide nanostructures.
Related collections
Most cited references236
- Record: found
- Abstract: not found
- Article: not found
Nanostructured Hydrogels for Three-Dimensional Cell Culture Through Self-Assembly of Fluorenylmethoxycarbonyl–Dipeptides
- Record: found
- Abstract: not found
- Article: not found
Fmoc-Diphenylalanine Self Assembles to a Hydrogel via a Novel Architecture Based on π–π Interlocked β-Sheets
- Record: found
- Abstract: found
- Article: not found