- Record: found
- Abstract: found
- Article: found
A prospective dual-centre intra-individual controlled study for the treatment of burns comparing dermis graft with split-thickness skin auto-graft
Read this article at
Abstract
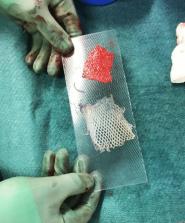
To investigate if donor and recipient site morbidity (healing time and cosmesis) could be reduced by a novel, modified split-thickness skin grafting (STSG) technique using a dermal component in the STSG procedure (DG). The STSG technique has been used for 150 years in surgery with limited improvements. Its drawbacks are well known and relate to donor site morbidity and recipient site cosmetic shortcomings (especially mesh patterns, wound contracture, and scarring). The Dermal graft technique (DG) has emerged as an interesting alternative, which reduces donor site morbidity, increases graft yield, and has the potential to avoid the mesh procedure in the STSG procedure due to its elastic properties. A prospective, dual-centre, intra-individual controlled comparison study. Twenty-one patients received both an unmeshed dermis graft and a regular 1:1.5 meshed STSG. Aesthetic and scar assessments were done using The Patient and Observer Scar Assessment Scale (POSAS) and a Cutometer Dual MPA 580 on both donor and recipient sites. These were also examined histologically for remodelling and scar formation. Dermal graft donor sites and the STSG donor sites healed in 8 and 14 days, respectively ( p < 0.005). Patient-reported POSAS showed better values for colour for all three measurements, i.e., 3, 6, and 12 months, and the observers rated both vascularity and pigmentation better on these occasions ( p < 0.01). At the recipient site, (n = 21) the mesh patterns were avoided as the DG covered the donor site due to its elastic properties and rendered the meshing procedure unnecessary. Scar formation was seen at the dermal donor and recipient sites after 6 months as in the standard scar healing process. The dermis graft technique, besides potentially rendering a larger graft yield, reduced donor site morbidity, as it healed faster than the standard STSG. Due to its elastic properties, the DG procedure eliminated the meshing requirement (when compared to a 1:1.5 meshed STSG). This promising outcome presented for the DG technique needs to be further explored, especially regarding the elasticity of the dermal graft and its ability to reduce mesh patterns.
Trial registration: ClinicalTrials.gov Identifier (NCT05189743) 12/01/2022.
Related collections
Most cited references25
- Record: found
- Abstract: found
- Article: not found
Demonstration of the safety and effectiveness of the RECELL® System combined with split-thickness meshed autografts for the reduction of donor skin to treat mixed-depth burn injuries
- Record: found
- Abstract: found
- Article: not found
Grafts in dermatologic surgery: review and update on full- and split-thickness skin grafts, free cartilage grafts, and composite grafts.
- Record: found
- Abstract: found
- Article: not found