- Record: found
- Abstract: found
- Article: found
Notch Signaling Limits Supporting Cell Plasticity in the Hair Cell-Damaged Early Postnatal Murine Cochlea
Read this article at
Abstract
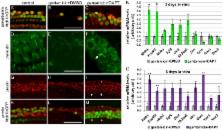
In mammals, auditory hair cells are generated only during embryonic development and loss or damage to hair cells is permanent. However, in non-mammalian vertebrate species, such as birds, neighboring glia-like supporting cells regenerate auditory hair cells by both mitotic and non-mitotic mechanisms. Based on work in intact cochlear tissue, it is thought that Notch signaling might restrict supporting cell plasticity in the mammalian cochlea. However, it is unresolved how Notch signaling functions in the hair cell-damaged cochlea and the molecular and cellular changes induced in supporting cells in response to hair cell trauma are poorly understood. Here we show that gentamicin-induced hair cell loss in early postnatal mouse cochlear tissue induces rapid morphological changes in supporting cells, which facilitate the sealing of gaps left by dying hair cells. Moreover, we provide evidence that Notch signaling is active in the hair cell damaged cochlea and identify Hes1, Hey1, Hey2, HeyL, and Sox2 as targets and potential Notch effectors of this hair cell-independent mechanism of Notch signaling. Using Cre/loxP based labeling system we demonstrate that inhibition of Notch signaling with a γ- secretase inhibitor (GSI) results in the trans-differentiation of supporting cells into hair cell-like cells. Moreover, we show that these hair cell-like cells, generated by supporting cells have molecular, cellular, and basic electrophysiological properties similar to immature hair cells rather than supporting cells. Lastly, we show that the vast majority of these newly generated hair cell-like cells express the outer hair cell specific motor protein prestin.
Related collections
Most cited references68
- Record: found
- Abstract: found
- Article: not found
A gene expression atlas of the central nervous system based on bacterial artificial chromosomes.
- Record: found
- Abstract: found
- Article: not found