- Record: found
- Abstract: found
- Article: found
Glucocorticoids induce senescence in primary human tenocytes by inhibition of sirtuin 1 and activation of the p53/p21 pathway: in vivo and in vitro evidence
Read this article at
Abstract
Cellular senescence is an irreversible side effect of some pharmaceuticals which can contribute to tissue degeneration.
Methods
Features of senescence (β-galactosidase activity at pH 6 (SA-β-gal) and active mammalian/mechanistic target of rapamycin (mTOR) in cell cycle arrest) as well as the activity of the two main pathways leading to cell senescence were examined in glucocorticoid-treated primary human tenocytes. Evidence of senescence-inducing pathway induction in vivo was obtained using immunohistochemistry on tendon biopsy specimens taken before and 7 weeks after subacromial Depo-Medrone injection.
Results
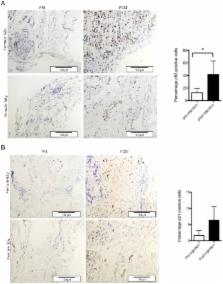
Dexamethasone treatment of tenocytes resulted in an increased percentage of SA-βgal-positive cells. Levels of phosphorylated p70S6K did not decrease with glucocorticoid treatment indicating mTOR remained active. Increased levels of acetylated p53 as well as increased RNA levels of its pro-senescence effector p21 were evident in dexamethasone-treated tenocytes. Levels of the p53 deacetylase sirtuin 1 were lower in dexamethasone-treated cells compared with controls. Knockdown of p53 or inhibition of p53 activity prevented dexamethasone-induced senescence. Activation of sirtuin 1 either by exogenous overexpression or by treatment with resveratrol or low glucose prevented dexamethasone-induced senescence. Immunohistochemical analysis of tendon biopsies taken before and after glucocorticoid injection revealed a significant increase in the percentage of p53-positive cells (p=0.03). The percentage of p21-positive cells also tended to be higher post-injection (p=0.06) suggesting glucocorticoids activate the p53/p21 senescence-inducing pathway in vivo as well as in vitro.
Related collections
Most cited references38
- Record: found
- Abstract: found
- Article: not found
Negative control of p53 by Sir2alpha promotes cell survival under stress.
- Record: found
- Abstract: found
- Article: not found
Human SIR2 deacetylates p53 and antagonizes PML/p53-induced cellular senescence.
- Record: found
- Abstract: found
- Article: not found