- Record: found
- Abstract: found
- Article: found
Co-targeting BCL-XL and BCL-2 by PROTAC 753B eliminates leukemia cells and enhances efficacy of chemotherapy by targeting senescent cells
Read this article at
Abstract
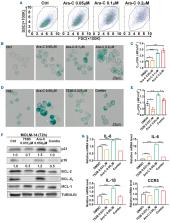
BCL-X L and BCL-2 are key anti-apoptotic proteins and validated cancer targets. 753B is a novel BCL-X L/BCL-2 proteolysis targeting chimera (PROTAC) that targets both BCL-X L and BCL-2 to the von Hippel-Lindau (VHL) E3 ligase, leading to BCL-X L/BCL-2 ubiquitination and degradation selectively in cells expressing VHL. Because platelets lack VHL expression, 753B spares on-target platelet toxicity caused by the first-generation dual BCL-X L/BCL-2 inhibitor navitoclax (ABT-263). Here, we report pre-clinical single-agent activity of 753B against different leukemia subsets. 753B effectively reduced cell viability and induced dose-dependent degradation of BCL-X L and BCL-2 in a subset of hematopoietic cell lines, acute myeloid leukemia (AML) primary samples, and in vivo patient-derived xenograft AML models. We further demonstrated the senolytic activity of 753B, which enhanced the efficacy of chemotherapy by targeting chemotherapy-induced cellular senescence. These results provide a pre-clinical rationale for the utility of 753B in AML therapy, and suggest that 753B could produce an added therapeutic benefit by overcoming cellular senescence-induced chemoresistance when combined with chemotherapy.
Related collections
Most cited references47
- Record: found
- Abstract: found
- Article: not found
The senescence-associated secretory phenotype: the dark side of tumor suppression.

- Record: found
- Abstract: found
- Article: found
Identification of a novel senolytic agent, navitoclax, targeting the Bcl‐2 family of anti‐apoptotic factors
- Record: found
- Abstract: found
- Article: not found