- Record: found
- Abstract: found
- Article: found
Dissecting the role of PfAP2-G in malaria gametocytogenesis
Read this article at
Abstract
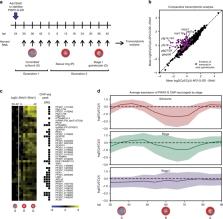
In the malaria parasite Plasmodium falciparum, the switch from asexual multiplication to sexual differentiation into gametocytes is essential for transmission to mosquitos. The transcription factor PfAP2-G is a key determinant of sexual commitment that orchestrates this crucial cell fate decision. Here we identify the direct targets of PfAP2-G and demonstrate that it dynamically binds hundreds of sites across the genome. We find that PfAP2-G is a transcriptional activator of early gametocyte genes, and identify differences in PfAP2-G occupancy between gametocytes derived via next-cycle and same-cycle conversion. Our data implicate PfAP2-G not only as a transcriptional activator of gametocyte genes, but also as a potential regulator of genes important for red blood cell invasion. We also find that regulation by PfAP2-G requires interaction with a second transcription factor, PfAP2-I. These results clarify the functional role of PfAP2-G during sexual commitment and early gametocytogenesis.
Abstract
The transcription factor PfAP2-G is a key determinant of sexual commitment in Plasmodium falciparum. Here, Josling et al. define the transcriptional regulatory network of PfAP2-G by identifying its DNA binding sites genome-wide, which vary depending on the route of sexual conversion and rely on interactions with the PfAP2-I transcription factor.
Related collections
Most cited references41
- Record: found
- Abstract: found
- Article: not found
A proteomic view of the Plasmodium falciparum life cycle.
- Record: found
- Abstract: found
- Article: not found
Genome-wide analysis of heterochromatin associates clonally variant gene regulation with perinuclear repressive centers in malaria parasites.
- Record: found
- Abstract: found
- Article: not found