- Record: found
- Abstract: found
- Article: found
Discovery of novel reversible inhibitor of DprE1 based on benzomorpholine for the treatment of tuberculosis
Read this article at
ABSTRACT
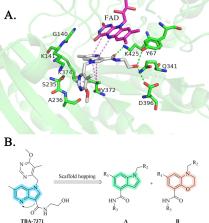
About a quarter of the world’s population is infected with Mycobacterium tuberculosis, equivalent to about two billion people. With the emergence of multidrug-resistant tuberculosis, those existing anti-tuberculosis drugs no longer meet the demand for cure anymore; there is an urgent need for the development of new anti-tuberculosis drugs. Decaprenylphosphoryl-β-D-ribose 2´-epimerase (DprE1) has been proven to be a potential antimycobacterial target, and several inhibitors have entered clinical trial. Herein, we designed and synthesized a series of compounds based on the indole and benzomorpholine by using the strategy of scaffold hopping. The preferred compound B18 showed strong antimycobacterial activity in H37Rv and drug-resistant clinical isolates. In addition, compound B18 did not exhibit antimycobacterial efficacy against other species of strains. Subsequently, the target of B18 was identified as DprE1 by analyzing spontaneous compound-resistant mutation data, and a docking study was performed to illustrate the binding mode between B18 and DprE1. In general, compound B18 is compatible to current DprE1 inhibitors, even higher phosphodiesterase 6C selectivity and plasma protein binding rate, which represent a new type of effective reversible DprE1 inhibitor.
IMPORTANCE
Drug therapy remains the cornerstone of tuberculosis (TB) treatment, yet first-line anti-tuberculosis drugs are associated with significant adverse effects that can compromise patient outcomes. Moreover, prolonged and widespread use has led to an alarming rise in drug-resistant strains of Mycobacterium tuberculosis, including multidrug-resistant [MDR-tuberculosis (TB)] and extensively drug-resistant (XDR-TB) forms. Urgent action is needed to develop novel anti-tuberculosis agents capable of overcoming these challenges. We report that compound B18, a decaprenylphosphoryl-β-D-ribose 2´-epimerase inhibitor with a benzomorpholine backbone, exhibits potent activity against not only the non-pathogenic strain H37Ra, but also the pathogenic strain H37Rv and clinical MDR and XDR strains. Preliminary druggability studies indicate that B18 possesses high safety and acceptable pharmacokinetic properties, rendering it a promising candidate for further development as a novel anti-tuberculosis agent.
Related collections
Most cited references19
- Record: found
- Abstract: found
- Article: not found
Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project.
- Record: found
- Abstract: found
- Article: not found