- Record: found
- Abstract: found
- Article: not found
Phosphorylation of the Kinase Domain Regulates Autophosphorylation of Myosin IIIA and Its Translocation in Microvilli
Read this article at
Abstract
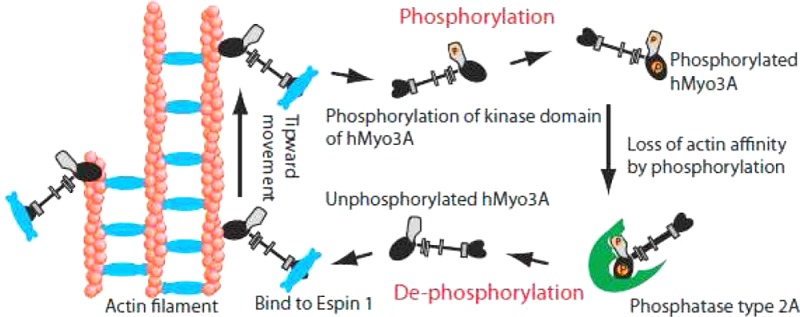
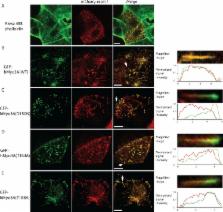
Motor activity of myosin III is regulated by autophosphorylation. To investigate the role of the kinase activity on the transporter function of myosin IIIA (Myo3A), we identified the phosphorylation sites of kinase domain (KD), which is responsible for the regulation of kinase activity and thus motor function. Using mass spectrometry, we identified six phosphorylation sites in the KD, which are highly conserved among class III myosins and Ste20-related misshapen (Msn) kinases. Two predominant sites, Thr 184 and Thr 188, in KD are important for phosphorylation of the KD as well as the motor domain, which regulates the affinity for actin. In the Caco2 cells, the full-length human Myo3A (hMyo3AFull) markedly enlarged the microvilli, although it did not show discrete localization within the microvilli. On the other hand, hMyo3AFull(T184A) and hMyo3AFull(T188A) both showed clear localization at the microvilli tips. Our results suggest that Myo3A induces large actin bundle formation to form microvilli, and phosphorylation of KD at Thr 184 and Thr 188 is critical for the kinase activity of Myo3A, and regulation of Myo3A translocation to the tip of microvilli. Retinal extracts potently dephosphorylate both KD and motor domain without IQ motifs (MDIQo), which was inhibited by okadaic acid (OA) with nanomolar range and by tautomycetin (TMC) with micromolar range. The results suggest that Myo3A phosphatase is protein phosphatase type 2A (PP2A). Supporting this result, recombinant PP2Ac potently dephosphorylates both KD and MDIQo. We propose that the phosphorylation–dephosphorylation mechanism plays an essential role in mediating the transport and actin bundle formation and stability functions of hMyo3A.
Related collections
Most cited references36
- Record: found
- Abstract: found
- Article: not found
The protein kinase family: conserved features and deduced phylogeny of the catalytic domains.
- Record: found
- Abstract: found
- Article: not found
Site-directed mutagenesis of double-stranded DNA by the polymerase chain reaction.
- Record: found
- Abstract: found
- Article: not found
