- Record: found
- Abstract: found
- Article: found
Aldolase A promotes epithelial‐mesenchymal transition to increase malignant potentials of cervical adenocarcinoma
Read this article at
Abstract
Recent studies have revealed that metabolic reprogramming is closely associated with epithelial‐mesenchymal transition (EMT) during cancer progression. Aldolase A (ALDOA) is a key glycolytic enzyme that is highly expressed in several types of cancer. In this study, we found that ALDOA is highly expressed in uterine cervical adenocarcinoma and that high ALDOA expression promotes EMT to increase malignant potentials, such as metastasis and invasiveness, in cervical adenocarcinoma cells. In human surgical specimens, ALDOA was highly expressed in cervical adenocarcinoma and high ALDOA expression was correlated with lymph node metastasis, lymphovascular infiltration, and short overall survival. Suppression of ALDOA expression significantly reduced cell growth, migration, and invasiveness of cervical cancer cells. Aldolase A expression was partially regulated by hypoxia‐inducible factor‐1α (HIF‐1α). Shotgun proteome analysis revealed that cell‐cell adhesion‐related proteins were significantly increased in ALDOA‐overexpressing cells. Interestingly, overexpression of ALDOA caused severe morphological changes, including a cuboidal‐to‐spindle shape shift and reduced microvilli formation, coincident with modulation of the expression of typical EMT‐related proteins. Overexpression of ALDOA increased migration and invasion in vitro. Furthermore, overexpression of ALDOA induced HIF‐1α, suggesting a positive feedback loop between ALDOA and HIF‐1α. In conclusion, ALDOA is overexpressed in cervical adenocarcinoma and contributes to malignant potentials of tumor cells through modulation of HIF‐1α signaling. The feedback loop between ALDOA and HIF‐1α could become a therapeutic target to improve the prognosis of this malignancy.
Abstract
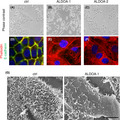
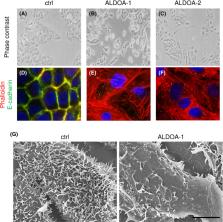
Aldolase A (ALDOA) overexpression caused epithelial‐mesenchymal transition‐like morphological alterations in cervical adenocarcinoma cells (A‐C). ALDOA‐overexpressing cells showed increased stress fiber formation (D‐F, red) and reduced E‐cadherin expression (D‐F, green) and microvilli formation (G).
Related collections
Most cited references43

- Record: found
- Abstract: found
- Article: found
Metabolic reprogramming: the emerging concept and associated therapeutic strategies
- Record: found
- Abstract: found
- Article: not found
The rising incidence of adenocarcinoma relative to squamous cell carcinoma of the uterine cervix in the United States--a 24-year population-based study.
- Record: found
- Abstract: found
- Article: not found