- Record: found
- Abstract: found
- Article: found
Design and Optimization of New Enteric Nanoparticles of Ceftriaxone for Oral Delivery: In vitro and in vivo Assessments
Abstract
Purpose
Development of new strategies for oral delivery of existing antibiotics administered exclusively through intravenous route is one of the global priorities of pharmaceutical research. The encapsulation of these active pharmaceutical agents within nanosized natural products offers several traits due to their tunable surface properties. Ceftriaxone (CTX) is an injectable, third-generation cephalosporin that suffers poor oral bioavailability.
Methods
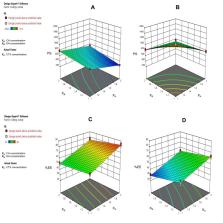
In the present study, ionic gelation of two biopolymers, namely chitosan (CH) and shellac (SH), was implemented to consolidate CTX, within elegant nanoparticles (NPs) for oral administration that would increase its bioavailability and sustainability. Quality by design approach (2 3 full factorial design) was adopted to optimize CTX-loaded nanoparticles. The optimized formula (F2) was characterized through transmission electron microscopy (TEM), Fourier transform infrared (FT-IR) spectroscopy and differential scanning calorimetry (DSC). In vitro release behavior and stability study were also evaluated. Pharmacokinetic studies of enteric-coated hard gelatin capsules (HGCs) loaded with F2-NPs were finally assessed.
Results
The optimized spherical F2-NPs had a mean particle size of 258 nm, zeta potential of about +30.1 and appreciable drug entrapment efficiency of 83%. The in vitro drug release profile of F2-NPs in pH 7.4 experienced biphasic configuration with an initial burst release for an hour, followed by a sustained release over 15 h with Higuchi model and non-Fickian diffusion mechanism (R 2=0.9852). High stability upon storage at refrigerated and room temperature for 3 months and good flow properties (θ= 32.2 and HR= 1.13) of the optimized formula were also conferred. In vivo pharmacokinetic assessment in rabbits fruitfully displayed 92% absolute bioavailability of CTX.
Graphical Abstract
Most cited references55
- Record: found
- Abstract: not found
- Article: not found
Mechanisms of solute release from porous hydrophilic polymers
- Record: found
- Abstract: not found
- Article: not found
MECHANISM OF SUSTAINED-ACTION MEDICATION. THEORETICAL ANALYSIS OF RATE OF RELEASE OF SOLID DRUGS DISPERSED IN SOLID MATRICES.

- Record: found
- Abstract: found
- Article: found
