- Record: found
- Abstract: found
- Article: found
Effects of Duodenal Infusion of Lauric Acid and L-Tryptophan, Alone and Combined, on Fasting Glucose, Insulin and Glucagon in Healthy Men
Read this article at
Abstract
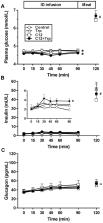
The fatty acid, lauric acid (‘C12’), and the amino acid, tryptophan (‘Trp’), when given intraduodenally at loads that individually do not affect energy intake, have recently been shown to stimulate plasma cholecystokinin, suppress ghrelin and reduce energy intake much more markedly when combined. Both fatty acids and amino acids stimulate insulin secretion by distinct mechanisms; fatty acids enhance glucose-stimulated insulin secretion, while amino acids may have a direct effect on pancreatic β cells. Therefore, it is possible that, by combining these nutrients, their effects to lower blood glucose may be enhanced. We have investigated the potential for the combination of C12 and Trp to have additive effects to reduce blood glucose. To address this question, plasma concentrations of glucose, insulin and glucagon were measured in 16 healthy, lean males during duodenal infusions of saline (control), C12 (0.3 kcal/min), Trp (0.1 kcal/min), or C12+Trp (0.4 kcal/min), for 90 min. Both C12 and C12+Trp moderately reduced plasma glucose compared with control ( p < 0.05). C12+Trp, but not C12 or Trp, stimulated insulin and increased the insulin-to-glucose ratio ( p < 0.05). There was no effect on plasma glucagon. In conclusion, combined intraduodenal administration of C12 and Trp reduced fasting glucose in healthy men, and this decrease was driven primarily by C12. The effects of these nutrients on postprandial blood glucose and elevated fasting blood glucose in type 2 diabetes warrant evaluation.
Related collections
Most cited references21
- Record: found
- Abstract: found
- Article: not found
Relationship between oral glucose tolerance and gastric emptying in normal healthy subjects.
- Record: found
- Abstract: found
- Article: not found
Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats.
- Record: found
- Abstract: found
- Article: not found