- Record: found
- Abstract: found
- Article: found
Increased Intestinal Epithelial Cell Turnover and Intestinal Motility in Gymnophalloides seoi-Infected C57BL/6 Mice
Read this article at
Abstract
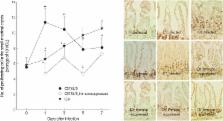
The changing patterns of goblet cell hyperplasia, intestinal epithelial cell turnover, and intestinal motility were studied in ICR and C57BL/6 mice infected with Gymnophalloides seoi (Digenea: Gymnophallidae). Whereas ICR mice retained G. seoi worms until day 7 post-infection (PI), C57BL/6 mice showed a rapid worm expulsion within day 3 PI. Immunosuppression with Depo-Medrol significantly delayed the worm expulsion in C57BL/6 mice. Goblet cell counts were increased in both strains of mice, peaking at day 1 PI in C57BL/6 mice and slowly increasing until day 7 PI in ICR mice. In C57BL/6 mice infected with G. seoi, newly proliferating intestinal epithelial cells were remarkably increased in the crypt, and the increase was the highest at day 1 PI. However, in ICR mice, newly proliferating intestinal epithelial cells increased slowly from day 1 to day 7 PI. Intestinal motility was increased in G. seoi-infected mice, and its chronological pattern was highly correlated with the worm load in both strains of mice. Meanwhile, immunosuppression of C57BL/6 mice abrogated the goblet cell proliferation, reduced the epithelial cell proliferation, and suppressed the intestinal motility. Goblet cell hyperplasia, increased intestinal epithelial cell turnover, and increased intestinal motility should be important mucosal defense mechanisms in G. seoi-infected C57BL/6 mice.
Related collections
Most cited references38
- Record: found
- Abstract: found
- Article: not found
Accelerated intestinal epithelial cell turnover: a new mechanism of parasite expulsion.
- Record: found
- Abstract: found
- Article: not found
Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites.
- Record: found
- Abstract: found
- Article: not found