- Record: found
- Abstract: found
- Article: not found
A Transcriptional Mechanism Integrating Inputs from Extracellular Signals to Activate Hippocampal Stem Cells
Read this article at
Summary
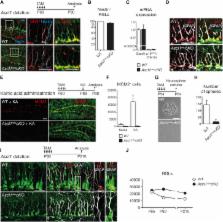
The activity of adult stem cells is regulated by signals emanating from the surrounding tissue. Many niche signals have been identified, but it is unclear how they influence the choice of stem cells to remain quiescent or divide. Here we show that when stem cells of the adult hippocampus receive activating signals, they first induce the expression of the transcription factor Ascl1 and only subsequently exit quiescence. Moreover, lowering Ascl1 expression reduces the proliferation rate of hippocampal stem cells, and inactivating Ascl1 blocks quiescence exit completely, rendering them unresponsive to activating stimuli. Ascl1 promotes the proliferation of hippocampal stem cells by directly regulating the expression of cell-cycle regulatory genes. Ascl1 is similarly required for stem cell activation in the adult subventricular zone. Our results support a model whereby Ascl1 integrates inputs from both stimulatory and inhibitory signals and converts them into a transcriptional program activating adult neural stem cells.
Highlights
-
•
Ascl1 is expressed specifically by activated stem cells of the hippocampus
-
•
Activating signals induce first Ascl1 expression and subsequently quiescence exit
-
•
Hippocampal stem cells expressing low levels of Ascl1 have reduced activity
-
•
Stem cells lacking Ascl1 remain permanently quiescent and unresponsive to stimuli
Abstract
Multiple extracellular signals regulate the activity of stem cells in the adult hippocampus. Andersen et al. show here that induction of the proneural protein Ascl1 in response to activation signals is absolutely required for stem cells to exit quiescence.
Related collections
Most cited references44
- Record: found
- Abstract: found
- Article: not found
Milestones of neuronal development in the adult hippocampus.
- Record: found
- Abstract: found
- Article: not found
Prospective identification and purification of quiescent adult neural stem cells from their in vivo niche.
- Record: found
- Abstract: found
- Article: not found
