- Record: found
- Abstract: found
- Article: found
Let-7a-5p may participate in the pathogenesis of diabetic nephropathy through targeting HMGA2
Read this article at
Abstract
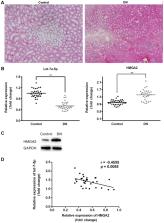
Diabetic nephropathy (DN) is one of the most common complications of diabetes mellitus (DM), and has been demonstrated as one of the major causes of renal failure. In a previous study, it was noted that microRNA let-7a-5p was downregulated in DN; however, the underlying mechanism requires additional investigation. The aim of the present study was to investigate the roles of let-7a-5p in the pathogenesis of DN and its associated mechanism. The renal tissues of db/db and db/m mice, and renal mesangial cells treated with high concentrations of glucose were obtained; reverse transcription-quantitative polymerase chain reaction, and western blot analysis were applied to detect the expression of let-7a-5p and high-mobility group AT-hook 2 (HMGA2) in vivo and in vitro. In addition, renal mesangial cells cultured under high-glucose conditions (20 and 30 mmol/l) were transfected with either let-7a-5p mimics or let-7a-5p inhibitors. The effects of let-7a-5p on the proliferation and apoptosis of renal mesangial cells were examined using CCK-8 and flow cytometry methods. Additionally, cells were collected and the expression of phosphoinositide 3-kinase (PI3K), phosphorylated protein kinase B (p-AKT) and HMGA2 was analyzed with western blot analysis. Finally, a dual luciferase reporter assay was performed to confirm whether HMGA2 was a direct target of let-7a-5p. Let-7a-5p was significantly downregulated and HMGA2 was markedly upregulated in the tissue samples of DN mice and renal mesangial cells cultured under high-glucose conditions. In addition, transfection of let-7a-5p mimics induced a significant decrease in the proliferation and increase in the apoptosis of renal mesangial cells cultured under high-glucose conditions; transfection of let-7a-5p inhibitors exhibited the opposite effects. Furthermore, transfection of let-7a-5p mimics also led to the inhibition of the PI3K-AKT signaling pathway; transfection of let-7a-5p inhibitors may activate the PI3K-AKT signaling pathway through the increase in PI3K and AKT levels. Finally, a dual luciferase reporter assay confirmed that HMGA2 is a direct target of let-7a-5p. Let-7a-5p was downregulated in DN and may participate in the pathogenesis of DN via regulating HMGA2 expression and the PI3K-AKT signaling pathway.
Related collections
Most cited references32
- Record: found
- Abstract: found
- Article: not found
Diabetic nephropathy: Is there a role for oxidative stress?

- Record: found
- Abstract: found
- Article: found
MicroRNA-27a promotes podocyte injury via PPARγ-mediated β-catenin activation in diabetic nephropathy
- Record: found
- Abstract: found
- Article: found